RHYTHMS: CIRCADIAN, INFRADIAN AND ULTRADIAN
CHRONOBIOLOGY
Chronobiology is the scientific study of biological rhythms.
More precisely:
Chronobiology examines how living organisms organise behaviour, physiology and internal processes according to daily (circadian), shorter (ultradian), and longer (infradian) cycles, and how these rhythms are generated and synchronised with the environment.
Core areas include:
• Circadian rhythms (about 24 hours)
• Ultradian rhythms (shorter cycles, e.g., sleep stages)
• Infradian rhythms (longer cycles, e.g., menstruation)
• Seasonal rhythms (e.g., migration, hibernation)
• Biological clocks (SCN, peripheral clocks)
• Zeitgebers (environmental cues like light or temperature)
• Entrainment (how internal rhythms align with the environment)
It bridges neuroscience, endocrinology, genetics, physiology and behaviour.
In plain terms:
Chronobiology studies how the body keeps time—and what happens when its internal clocks drift, misalign or are disrupted
WHY CHRONOBIOLOGY MATTERS IN PSYCHOLOGY
Chronobiology explains real-world phenomena such as shift-work disorder, jet lag, seasonal affective disorder (SAD), teenage sleep-phase delay, and the timing of medication (chronotherapy). Questions on “applications”, “issues and debates” (nature–nurture, reductionism), and “research methods” (e.g., actigraphy, constant routines) frequently draw on chronobiology.
Make it stand out
Whatever it is, the way you tell your story online can make all the difference.
SPECIFICATION: Biological rhythms: circadian, infradian, and ultradian, and the differences between these rhythms. The effect of endogenous pacemakers and exogenous zeitgebers on the sleep/wake cycle
The specification outlines several requirements for this topic.
Students are expected to:
Describe and identify the three primary biological rhythms—circadian, ultradian and infradian
Apply this knowledge when explaining aspects of behaviour.
Understand the roles of endogenous pacemakers and exogenous zeitgebers.
Explain how these internal and external factors regulate biological timing.
Beyond description, students are required to evaluate these mechanisms by assessing the quality of the supporting evidence, identifying methodological weaknesses in the research, and assessing how effectively each mechanism accounts for human behaviour.
Although this content appears toward the end of the specification, it is conceptually extensive because it integrates multiple components: biological rhythms, internal timing systems and environmental cues. Many evaluation points and research studies can be applied across different parts of the topic, which makes the material more coherent once the underlying processes are understood. A firm grasp of how each rhythm and regulatory system functions is essential before advanced discussion and evaluation can be carried out effectively
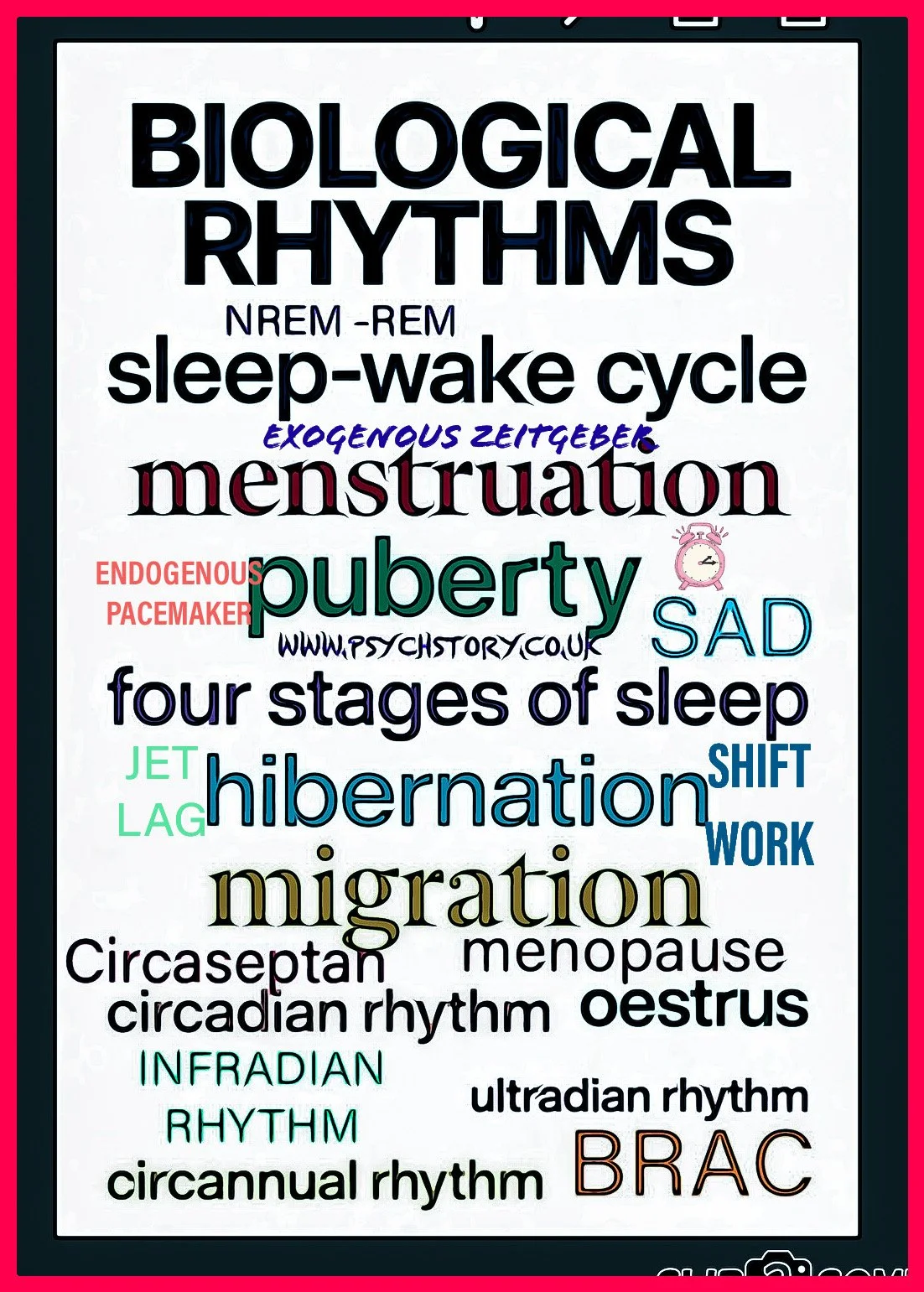
KEY TERMS – BIOLOGICAL RHYTHMS (ALPHABETICAL ORDER)
ROOT TERMS FOR BIOLOGICAL RHYTHMS
Circa = about
Diem = day
Ultra = beyond (more frequent than once per day)
Infra = below (less frequent than once per day)
Annus = year
Zeit is German for “time.”
(From Zeit = time.)Geber means “giver.”
Zeitgeber literally means: “time giver” → something that gives the organism its timing cue. Any external cue—e.g., light—that resets and synchronises the body’s internal biological clock.
Pacemaker =A mechanism that generates and regulates rhythmic activity; in humans, it refers to internal clocks, such as the suprachiasmatic nucleus (SCN), that control circadian rhythms.
Endogenous: Originating within the body; describes internal biological mechanisms that regulate rhythms independently of external cues.
Exogenous: Originating outside the body; refers to environmental or external factors that influence and reset internal biological clocks.
Exogenous Zeitgeber =An external time cue that resets internal biological clocks.
Exogenous Pacemaker = An internal biological clock
ADENOSINE
a neurotransmitter that accumulates during wakefulness, increasing sleep pressure; cleared during sleep.AMPLITUDE
The height of a brain wave on an EEG reflects how synchronised neuronal firing is.ANNUS
Latin root meaning “year.”ARAS (ASCENDING RETICULAR ACTIVATING SYSTEM)
brainstem arousal network promoting wakefulness; part of the ultradian sleep–wake cycling mechanism.BIOLOGICAL RHYTHM:
Any repeating pattern of physiological or behavioural activity.BRAIN WAVE
The electrical activity of the brain, measured by EEG, varies by sleep stage and frequency.CHRONO
Greek root meaning “time.”CHRONOBIOLOGY:
The study of biological rhythms and internal timing systems.CHRONOPHARMACOLOGY:
The study of how drug effects vary with the timing of administration.CIRCADIAN RHYTHM
A biological rhythm lasting about 24 hours (e.g., sleep–wake cycle).CIRCA
Latin root meaning “about.”CORTISOL
a hormone peaking in the early morning to promote alertness.DIEM
Latin root meaning “day.”EEG (ELECTROENCEPHALOGRAM)
measures electrical brain activity via scalp electrodes.ENTRAINMENT
The process by which external cues synchronise internal rhythms to the environment.FREE-RUNNING RHYTHM
the natural rhythm expressed without zeitgebers (e.g., constant darkness).HPA AXIS (HYPOTHALAMIC–PITUITARY–ADRENAL)
a system controlling stress responses; influences cortisol and circadian stability.HPO AXIS (HYPOTHALAMIC–PITUITARY–OVARIAN)
regulates the menstrual cycle; it is the endogenous pacemaker for infradian rhythms.INFRA
Latin root meaning “below.”INFRADIAN RHYTHM:
A rhythm lasting longer than 24 hours (e.g., the menstrual cycle).JET LAG
A mismatch between internal circadian timing and local time after travel.LUX
A unit of light intensity.MELATONIN
a hormone released in darkness to promote sleep.NON-24-HOUR SLEEP–WAKE DISORDER
A condition where the circadian clock cannot be reset by light (common in total blindness).PACEMAKER
any internal system generating regular rhythmic activity (SCN, HPO axis, VLPO/brainstem circuits).PERIPHERAL CLOCKS
clock genes found in organs outside the SCN (e.g., liver, heart).PHARMACOKINETICS: The action of drugs on the body and how well they are absorbed and distributed
PHEROMONES
chemical signals that may influence physiology or behaviour in members of the same species; evidence for human menstrual synchrony is weak.PHOTORECEPTORS
Light-sensitive retinal cells that send light information to the SCN.PULSATILE RELEASE PATTERN
Hormones are released in bursts rather than continuously.RAPID EYE MOVEMENT (REM)
a sleep stage characterised by mixed-frequency brain waves and vivid dreaming.REM-ON / REM-OFF NEURONS
Brainstem neurons that switch REM sleep on and off; essential for the ultradian cycling of REM and NREM sleep.ROOT TERMS
Basic conceptual prefixes (circa, diem, ultra, infra, annus).SCN (SUPRACHIASMATIC NUCLEUS)
A cluster of neurons in the hypothalamus that acts as the master circadian pacemaker.SEROTONIN
A neurotransmitter involved in mood and sleep; precursor to melatonin.SHIFT WORK
Misaligned employment patterns can cause fatigue and health issues.SLEEP–WAKE CYCLE
The primary circadian rhythm controls daily patterns of sleep and wakefulness.SYMPATHETIC AROUSAL
Activation of the sympathetic nervous system during stress can disrupt circadian and infradian rhythms.TAU (τ)
The intrinsic length of the free-running circadian period (slightly more than 24 hours in humans).THALAMUS
a relay centre that filters sensory information during sleep, preventing unnecessary arousal.ULTRA
Latin root meaning “beyond.”ULTRADIAN RHYTHM
A rhythm shorter than 24 hours (e.g., the 90-minute sleep cycle).VLPO (VENTROLATERAL PREOPTIC NUCLEUS)
a hypothalamic region that promotes sleep by inhibiting arousal systems; part of the ultradian pacemaker network.
BIOLOGICAL RHYTHMS
IN SHORT
Biological rhythms are biological processes that exhibit cyclical variation over time, ranging from hours to years, and reflect the influence of Earth's rotation on us… It's living inhabitants, along with plants and animals.
There are three rhythms we will focus on throughout this module: Circadian, Infradian, and ultradian biological rhythms.
IN MORE DEPTH
Biological rhythms are the body’s natural cycles that organise when various physical and behavioural processes occur. They prevent random events by providing the body with a predictable pattern to follow. These rhythms come from internal systems that keep time and allow essential activities—such as growth, repair, sleep, and hormone release—to occur in the correct order.
These rhythms run over different lengths of time. Some are monthly, such as the menstrual cycle, which follows a repeating pattern of hormonal changes. Others unfold over years, such as puberty, when hormone levels gradually rise, triggering significant physical and emotional changes. Many animals follow yearly rhythms, such as migration or hibernation, that help them survive seasonal changes. There are also very short rhythms: during the night, the NREM–REM sleep cycle repeats in a regular sequence of deep sleep, light sleep, and dreaming. Even the daily sleep–wake pattern shows how the body moves through a familiar cycle of alertness and rest.
Together, these examples show that biological rhythms are real, observable patterns. They help the body stay organised and adapt to the world, rather than functioning as a set of disconnected processes.
NEUROSCIENCE AND ENDOCRINOLOGY
Biological rhythms depend on communication between the brain and the endocrine system. The brain provides the timing mechanism, ensuring that cycles such as sleep, hunger, and alertness occur in a regular pattern. The endocrine system provides chemical control, using hormones to initiate, maintain, and terminate each process. For example, the brain signals the release of melatonin to trigger sleep, cortisol to promote wakefulness, and growth hormone during deep sleep for physical repair. Together, these systems allow the body to regulate its internal timing and maintain stable biological rhythms.
TYPES OF BIOLOGICAL RHYTHMS
CIRCADIAN RHYTHMS: From Latin circa = about, diem = day. Circadian rhythms are the body’s built-in 24-hour cycles.
EXAMPLES:
Sleep–wake cycle – The daily rhythm of alertness and sleepiness, regulated by the SCN and melatonin. We feel awake in daylight and sleepy at night because the circadian system works on a 24-hour timetable.
Core body temperature cycle – Body temperature rises during the day and falls at night, following a predictable daily pattern that affects alertness, concentration and cognitive performance.
Hormonal rhythms (e.g., cortisol) – Cortisol peaks in the early morning to support wakefulness and gradually declines across the day. This daily rise and fall is a clear circadian pattern.
Patterns of alertness and reaction time – Humans show a daily rhythm in cognitive performance, with faster reaction times in the late afternoon and slower responses during the night, reflecting underlying circadian processes.
ULTRADIAN RHYTHMS The word ultra means from Latin ultra = beyond, describing rhythms that occur more than once in 24 hours. These are short cycles that repeat several times each day or night, e.g., more than once within 24 hours. They are brief, recurring patterns in physiological and behavioural activity that operate continuously throughout the day and/ or night.
EXAMPLES:
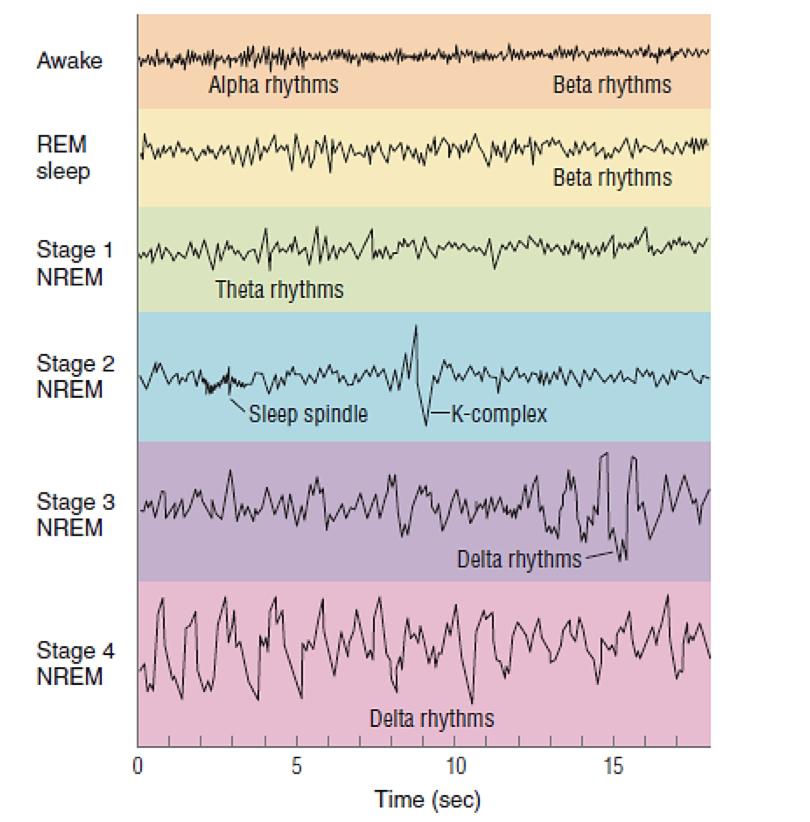
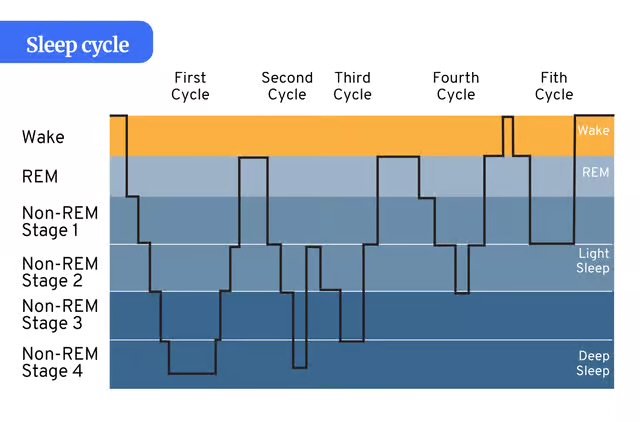
The NREM REM sleep cycle – the 90-minute pattern of NREM and REM sleep repeated multiple times per night.
Heart rate and breathing patterns – continuous, short-term physiological rhythms.
Hormone pulsatility – growth hormone, insulin, and cortisol are released in bursts throughout the day.
Basic Rest–Activity Cycle (BRAC) – 90-minute cycles of alertness and fatigue are observable even during waking hours.
INFRADIAN RHYTHMS: From Latin infra = below) The word infra means “below”, referring to rhythms that occur less frequently than once every 24 hours. They are longer-term cycles extending over days, weeks, or months.
EXAMPLES:
The menstrual cycle – A monthly rhythm in females regulated by hormones that prepares the uterus for pregnancy. Approximately 28 days, controlled by changes in oestrogen and progesterone.
The biannual oestrus cycle – A seasonal reproductive cycle in many mammals, repeating twice per year, and therefore an infradian rhythm because each cycle lasts far longer than twenty-four hours but shorter than a year.
CIRCANNUAL RHYTHMS(from Latin circa = about, annus = year)
These rhythms operate on roughly a yearly cycle, often tied to environmental changes such as daylight length or temperature.
EXAMPLES:
Hibernation – reduced activity and metabolic rate during winter.
Migration – seasonal travel of birds and animals for food or breeding.
Reproductive timing – breeding patterns aligned with favourable conditions.
The annual oestrus cycle (in two categories) – A seasonal reproductive cycle in many mammals, which repeats once per year instead of twice per year or once per month, and is a circannual rhythm because each cycle lasts far longer than twenty-four hours but shorter than a year.
Seasonal affective disorder (SAD): A yearly pattern where individuals experience lower mood and energy during the winter months, linked to reduced daylight exposure. Common in winter in the northern hemisphere.
SUMMARY
Ultradian → repeats many times in a day (< 24h)
Circadian → repeats every 24h
Infradian → takes > 24h to repeat (eg, 48h, weekly, monthly, seasonal)
Circannual → roughly 1 year
ENDOGENOUS PACEMAKERS AND EXOGENOUS ZEITGEBERS
KEY TERMS
PACEMAKER
A mechanism that generates and regulates rhythmic activity; in humans, it refers to internal clocks, such as the suprachiasmatic nucleus (SCN), that control circadian rhythms.ZEITGEBER
German for “time giver.” Any external cue—exceptionally light—that resets and synchronises the body’s internal biological clockENDOGENOUS
Originating within the body, it describes internal biological mechanisms that regulate rhythms independently of external cues.EXOGENOUS
Originating outside the body, it refers to environmental or external factors that influence and reset internal biological clocks.
ENDOGENOUS PACEMAKERS & EXOGENOUS ZEITGEBERS – THE CORE OF ALL BIOLOGICAL RHYTHMS
All biological rhythms (daily sleep-wake, stages of sleep, menstrual cycle, seasonal changes, etc.) depend on two things working together:
Endogenous pacemakers – these are the internal biological clocks inside us (the main one is the SCN in the brain, plus clocks in almost every organ and cell). They keep our bodies running on roughly 24-hour cycles even when there are no external cues.
Exogenous zeitgebers – these are the external time cues from the environment: light-dark cycle, meal times, exercise, temperature, and social routines. Their job is to grab the internal clocks and adjust them so they match the real world exactly.
Why you absolutely need to know them
Every single thing you will ever be asked about biological rhythms comes back to a straightforward question:
“Are the internal clocks and the external cues properly lined up, or are they out of step?”
Jet lag = cues changed too fast
Shift work = cues flipped upside-down
Teenagers sleeping late = internal clock naturally shifted
Medicine working better at certain times = using cues to hit the clock at the right phase
Get these two ideas straight, and the whole topic suddenly makes perfect sense. That’s why they’re the first (and most important) thing to learn.
ENDOGENOUS PACEMAKERS
Endogenous pacemakers are the body’s internal biological timing systems that generate, maintain and regulate the pattern of biological rhythms. These mechanisms operate within the organism and continue to create rhythmic activity even in the absence of external cues. Each pacemaker controls the timing of a rhythm on its own timescale. For short cycles, such as ultradian rhythms, internal oscillators within the brainstem and autonomic nervous system regulate repeated patterns of activity and rest. For daily rhythms, circadian timing systems set the 24-hour pattern of physiological and behavioural changes. For longer rhythms, such as infradian and circannual cycles, internal hormonal and neural systems organise monthly reproductive patterns or seasonal changes in behaviour and physiology.
Although endogenous pacemakers differ across timescales, they share a fundamental role: they provide a predictable internal structure that organises bodily functions, ensuring that events such as sleep, hormone release, reproduction, and long-term seasonal behaviours occur in a coordinated sequence. These timing systems continue to run in the absence of external information, but their accuracy can drift. This shows that endogenous pacemakers generate the rhythm, while environmental cues are needed to keep it aligned with the outside world.
APPLYING PACEMAKERS TO CIRCADIAN RHYTHMS
In circadian rhythms, the primary endogenous pacemaker is the suprachiasmatic nucleus (SCN) in the hypothalamus. The SCN generates a roughly 24-hour rhythm that regulates processes such as body temperature, hormone release and the sleep–wake cycle. Even in constant darkness, the SCN continues to produce a near-daily rhythm, demonstrating that circadian timing originates from an internal biological clock.
EXAMPLES OF ENDOGENOUS PACEMAKERS
CIRCADIAN RHYTHM – The sleep-wake cycle. The SCN (suprachiasmatic nucleus) is the internal body clock that generates the 24-hour rhythm.
Pineal gland + melatonin – releases melatonin at night, promoting sleep.
INFRADIAN RHYTHM The menstral Cycle: Hypothalamic–pituitary–ovarian (HPO) axis – the internal hormonal system that sets the timing of the menstrual cycle.
ULTRADIAN RHYTHM: Brainstem sleep mechanisms (pons, medulla) – generate the 90-minute REM/NREM cycle.
Thalamus – controls NREM rhythms.
CIRCANNUAL RHYTHM: Internal seasonal clock in the hypothalamus – responds to changes in day length and prepares animals for long-term cycles like hibernation.
Melatonin patterns across seasons – more extended melatonin release in winter signals the season.
EXOGENOUS ZEITGEBERS
DEFINTION
Exogenous zeitgebers are environmental signals that influence the timing of biological rhythms. These cues provide reliable information about changes in the external world, and the body uses this information to adjust internal pacemakers so that biological rhythms stay synchronised with the environment.
They influence all types of rhythms, regardless of timescale. Light and temperature help animals time their circannual behaviours such as hibernation and migration. Social routines, food availability and daily activity patterns influence circadian rhythms such as sleep–wake timing. Mealtimes, stimulation levels and evening habits shape ultradian rhythms such as the progression of REM and NREM sleep. Longer environmental patterns, including seasonal changes, can affect infradian rhythms such as reproductive cycles.
Without exogenous zeitgebers, these rhythms continue because they are generated internally, but they often drift or become poorly aligned with real-world conditions. This misalignment shows that the body relies on exogenous signals not simply as supplementary information but as active mechanisms for entrainment, allowing internal timing systems to remain accurate and adaptive across the different biological timescales.
APPLYING EXOGENOUS ZEITGEIBERS TO CIRCADIAN RHYTHMS
In circadian rhythms, the most crucial zeitgeber is light. Light signals detected by the eyes provide daily information about morning and evening, helping the internal circadian pacemaker to stay aligned with the 24-hour day–night cycle. Other cues, such as mealtimes, activity patterns and social routines, also help stabilise the sleep–wake cycle by reinforcing when the body should be alert and when it should rest.
Other important exogenous zeitgebers here include:
Social cues such as mealtimes, work schedules, and social interaction.
Temperature changes, which signal the time of day and influence metabolic rate.
Routine behaviours like exercise or exposure to artificial light.
Without these cues, the body’s internal rhythm can drift, leading to free-running cycles slightly longer or shorter than 24 hours. This shows that exogenous zeitgebers are essential for entrainment—the process of aligning internal biological rhythms with the external world.
EXAMPLE OF EXOGENOUS ZEITGEBERS
CIRCADIAN RHYTHM:
Light – the primary external cue that resets the SCN each day.
Social routines – mealtimes, activity patterns, work/school times.
Exercise & daily activity – helps stabilise the rhythm.
INFRADIAN RHYTHM:
Pheromones – possible influence when women live closely together.
Stress – can delay or disrupt the cycle.
Nutrition and energy levels – under-eating or sudden weight change can alter cycle timing.
ULTRADIAN RHYTHM:
Cannabis alters REM density and can reduce REM sleep, affecting the pattern of ultradian cycling.
Alcohol disrupts REM in the second half of the night and fragments the 90-minute cycle.
Benzodiazepines – reduce deep NREM sleep and change the structure of REM/NREM cycles.
Caffeine – delays sleep onset and suppresses deep sleep, shifting the timing of ultradian cycles.
Noise and environmental stimulation – interrupt the natural cycling between NREM and REM.
Light before sleep suppresses melatonin and alters the first REM period.
Evening routines and activity levels influence sleep onset and the stability of REM/NREM cycles
CIRCANNUAL RHYTHM:
Day length (photoperiod) – the primary cue signalling winter or summer.
Temperature – falling temperatures cue hibernation behaviour.
Food availability – declining food supply triggers preparation for hibernation.
Identical twins are born in Tromsø, Norway, in 1987. A hospital mix up means they are swapped at birth and raised by completely different families.
Zeit grows up with a Sami reindeer herder far above the Arctic Circle. There is never a clock in the house. The only thing that ever tells him when it should be day or night is natural light. In summer the sun never sets for about two months. There is no night. Zeit can hardly sleep and ends up completely exhausted. In winter the sun never rises for about two months. There is no day. Zeit sleeps sixteen to eighteen hours and can barely stay awake when he finally gets up.
Nate grows up with a retired sea captain who runs everything by one old ship’s clock from the nineteen forties. The clock has never been repaired and now takes exactly twenty six hours to complete one full day. Nate always gets eight perfect hours of sleep, but because he has only ever followed that clock that cannot keep time, his sleeping and waking times shift roughly two hours later every real twenty four hour day, so he eventually tries to milk his cows at three in the morning.
The TV reunion is booked for Saturday eight p.m. Zeit arrives on the right evening, pale and shaking from weeks without a proper night. Nate checks the only clock he has ever known, puts on a suit, and arrives at the studio at two thirty six a.m. on Tuesday morning, wondering where everyone is.
Here is the real point. In real life the sleep wake cycle depends on two forces. The internal body clock sets the rhythm, but like Zeit’s situation it does not run to exactly twenty four hours. It runs slightly long. Every morning natural light gives the correcting cue and drags that longer internal rhythm back to a true twenty four hour day.
We need both: the inner clock that generates the cycle and the daily light that keeps it aligned with the real world. We call this circadian entrainment.
DIFFERENT TYPES OF BIOLOGICAL RHYTHMS IN DEPTH
CIRCADIAN RHYTHMS:
CIRCADIAN RHYTHMS
(from Latin circa = about, diem = day)
DEFINITION
Circadian rhythms are the body’s built-in 24-hour cycles that keep physical, mental, and emotional processes running in time with the Earth’s day–night pattern. The name comes from circa diem, meaning “about a day.” You can think of them as the body’s internal timetable. This biological clock quietly keeps everything in sync, much like a conductor coordinating an orchestra so that every instrument plays at the right moment.
Each organ and cell in the body contains its own microscopic biological clock. Still, all of them take their cue from one “master clock” in the brain — the suprachiasmatic nucleus (SCN), located in the hypothalamus. The SCN is made up of about 20,000 neurons that keep time through rhythmic electrical activity. Its job is to synchronise all the more miniature clocks in the body so that different systems — such as digestion, temperature regulation, and hormone release — follow the same daily rhythm.
Without this coordination, the body’s internal processes would drift out of sync with one another — a bit like a band with every musician playing to a different beat. You might find your digestive system demanding lunch at 3 a.m. while your sleep system insists on a nap in the supermarket queue. Body temperature might peak when you’re trying to fall asleep, and your alertness levels could hit their high point just as you climb into bed. In short, you’d be permanently jet-lagged without ever leaving your house — the kind of person who could fall asleep anywhere, anytime, except when it’s actually bedtime.
WHAT MAKES A RHYTHM “CIRCADIAN”?
For a biological rhythm to be classed as circadian, it must meet three main conditions:
1. IT MUST BE INTERNAL (ENDOGENOUS)
The rhythm continues even without external cues like light or temperature. For example, if an organism is kept in constant darkness, its body clock still runs on roughly a 24-hour cycle — called the free-running period (symbol τ, “tau”). In humans, this period is usually just over 24 hours. This shows that circadian rhythms arise within the body, not just from responding to the environment.
2. IT MUST BE RESET BY EXTERNAL CUES (ENTRAINABLE)
Although the rhythm is internal, it can be adjusted by zeitgebers (“time givers”) such as light, temperature, or social cues. This keeps the body clock in sync with the real world. For example, when someone flies to another time zone, they experience jet lag until their circadian clock entrains to the new local time.
3. IT MUST BE TEMPERATURE COMPENSATED
The rhythm stays close to 24 hours even when the body or environmental temperature changes. This prevents the body clock from speeding up in the heat or slowing down in the cold. Scientists call this temperature compensation, and they measure it with a value called Q10. If Q10 stays near 1, it means the rhythm keeps good time despite temperature changes.
In short, circadian rhythms are internal, can be reset, and stay stable across temperatures — allowing living organisms to keep accurate time in a changing world.
CIRCADIAN RHYTHM:THE SLEEP-WAKE CYCLE
ENDOGENOUS PACEMAKER FOR THE SLEEP WAKE CYCLE: THE SCN
The SCN keeps this chaos in check by receiving light information from the eyes. Special photoreceptor cells in the retina detect changes in brightness and send this data along the retinohypothalamic tract to the SCN. This allows the brain to track day and night even with the eyes closed, ensuring the body doesn’t start its “day shift” at midnight.
Once the SCN knows it’s morning, it communicates with other brain regions to control hormone release. Its main target is the pineal gland, which produces melatonin, the hormone that signals “sleep time.” When daylight enters the eyes, the SCN sends inhibitory signals to the pineal gland, reducing melatonin production, while simultaneously increasing serotonin release, which enhances mood, focus, and wakefulness. This is why getting sunlight in the morning can lift energy and improve alertness.
As evening arrives and light levels drop, the SCN flips the switch. It stimulates the pineal gland to release melatonin, which makes you sleepy and ready to wind down. As darkness falls, melatonin levels rise and core body temperature drops, preparing the body for rest. Even in complete darkness, these cycles continue for a time, showing that they are internally generated — but without external light cues, they eventually drift out of sync. This is why we feel jet lag when travelling across time zones or struggle to sleep after using bright screens late at night. Melatonin also acts on the raphe nuclei — clusters of neurons in the brainstem that regulate serotonin — slowing brain activity and promoting relaxation.
This seesaw between serotonin by day and melatonin by night keeps you balanced: energetic and focused when the world is bright, tired and calm when it’s dark. Thanks to the SCN, your body doesn’t have to guess when to eat, move, or sleep — it already knows. But when this clock is thrown off, as in night shifts or scrolling on your phone at 2 a.m., your brain ends up thinking it’s sunrise and wonders why you’re lying down instead of making breakfast.
DAMAGE TO THE SCN
When the SCN (suprachiasmatic nucleus) is damaged or disconnected from light cues, circadian rhythms break down.
Key effects
Loss of 24-hour rhythm: Sleep–wake cycles, body temperature, and hormone secretion (e.g. melatonin, cortisol) become irregular or completely arrhythmic.
Free-running rhythms: If the SCN still functions but is isolated from light input, internal rhythms continue but drift out of sync with the external day–night cycle (about 24.2 hours in humans).
Animal studies: Lesions of the SCN in hamsters and rats abolish normal activity–rest cycles; transplanting an SCN from another animal restores rhythmicity.
In short, the SCN is the master clock. Damage destroys internal timing; isolation from light cues prevents entrainment to the environment.
SUMMARY
In essence, circadian rhythms act as the body’s master scheduling system. They coordinate countless processes — sleep, digestion, metabolism, alertness, and even cell repair — so that the body functions smoothly and efficiently across the 24-hour cycle.
Circadian rhythms regulate essential functions, including sleep, hormone release, metabolism, mood, and cognitive performance. They help the body anticipate regular environmental changes rather than merely reacting to them. When circadian rhythms are disrupted—through jet lag, shift work, or excessive screen exposure—the result can be fatigue, poor concentration, mood disturbance, and long-term health effects such as metabolic and cardiovascular problems.
OTHER EXAMPLES OF CIRCADIAN RHYTHMS
Circadian rhythms also control other bodily processes that follow a daily rhythm. Body temperature is lowest in the early morning and highest in the early evening; blood pressure rises during the day and falls at night; and hormones such as cortisol peak around the time of waking to prepare the body for activity. Together, these patterns create a biological rhythm that matches our internal state to the external environment..
URINE PRODUCTION
Decreases at night to reduce sleep disturbance; increases during the day when activity levels rise, showing circadian control of kidney function.
DIGESTIVE ACTIVITY
The stomach and intestines show rhythmic activity, with peak acid secretion, enzyme release, and nutrient absorption during the day when eating is most likely.
LIVER METABOLISM
The liver’s detoxification and glucose regulation enzymes fluctuate across the 24-hour cycle, affecting how drugs and nutrients are processed.
CELL REPAIR AND DNA SYNTHESIS
Rates of tissue repair and DNA replication peak at night, indicating circadian regulation of cellular maintenance and regeneration.
IMMUNE FUNCTION
Activity of immune cells, such as lymphocytes and cytokines, varies throughout the day, with many immune responses heightened at night.
HORMONE RELEASE (GENERAL)
In addition to melatonin and cortisol, other hormones—including growth hormone—follow circadian rhythms, with secretion highest during early sleep to promote physical recovery and growth.
These examples show that while the sleep–wake cycle remains the core circadian rhythm, the SCN coordinates a vast network of peripheral rhythms across organs and systems, maintaining 24-hour balance in metabolism, repair, and behaviour.
RESEARCH STUDIES ON CIRCADIAN RHYTHMS
Understanding of circadian disruption has been advanced through a range of different research methods, including case studies, lesion studies, transplant experiments, genetic and molecular research, and controlled human laboratory studies. Together, these approaches trace the brain’s internal timing mechanisms and reveal their physiological consequences. Research across several decades has shown that the suprachiasmatic nucleus (SCN), its hormonal pathways, and underlying clock genes regulate daily biological rhythms — and that irregular light exposure, shift work, or jet lag can interfere with them.
SIFFRE AND OTHER ISOLATION STUDIES
SIFFRE (1962, 1975, 1999)
Michel Siffre conducted a series of influential self-experiments in sensory isolation to investigate whether human circadian rhythms continue without external time cues.
In his first study (1962) in the Alps, he lived in a cave for two months with no natural light, clocks, or social contact. When he resurfaced, he believed the date was weeks earlier than it actually was. His internal sense of time had drifted, and his sleep–wake cycle lengthened to around 25 hours, showing that biological rhythms continue but do not match the 24-hour day without environmental cues.
His second study (1975) was more controlled and lasted six months. Siffre lived alone in a Texas cave with regular monitoring via telephone. All external zeitgebers were removed. His free-running rhythm again settled slightly longer than 24 hours, sometimes drifting towards 30–48 hours. Periods of wakefulness extended and sleep onset became inconsistent. This reinforced the idea that humans possess a robust endogenous pacemaker capable of generating its own rhythm, but it is not perfectly aligned with the natural day-night cycle.
In his later study (1999), Siffre repeated the isolation experiment at age sixty. His circadian rhythm became even longer and more unstable, suggesting that the internal clock changes with age. Drift was greater, sleep became fragmented, and his rhythm fluctuated more widely. This provided rare evidence that endogenous pacemakers weaken or become less precise across the lifespan.
Across all studies, Siffre demonstrated that:
• humans have an internal biological clock;
• this clock persists without cues;
• it is not precisely 24 hours;
• without zeitgebers, the rhythm drifts, becomes unstable, and loses synchrony
CRITICISMS AND LIMITATIONS OF SIFFRE’S RESEARCH
Although Michel Siffre’s isolation studies (1962, 1975, 1999, 2007) were pioneering in demonstrating the existence of an endogenous circadian rhythm, they suffer from several critical methodological and interpretative weaknesses.
LACK OF GENERALISABILITY
A significant issue is that Siffre acted as both the researcher and sole participant in his early experiments. His findings, therefore, lack population validity and cannot be generalised across variables such as age, gender, or lifestyle. Later in life, Siffre repeated the procedure at the age of 60 and found his rhythm slowed considerably, suggesting that circadian timing changes with age. This further highlights the problem of using a single participant — the results may reflect individual variation rather than universal biological principles.ARTIFICIAL LIGHT AND LUX LEVELS
Siffre’s claim that his rhythm was “free-running” has been vigorously challenged by later research into light intensity (lux). Despite living in isolation from natural cues, he still used artificial lighting to see and work, assuming dim light had no biological effect. However, Czeisler et al. (1999) found that even low-intensity light (150–200 lux) can shift circadian phase and suppress melatonin, whereas ordinary daylight ranges from 10,000 to 100,000 lux. This means the “dim” lighting in Siffre’s cave was still bright enough to act as a weak zeitgeber, resetting his internal clock. His reported 25-hour rhythm may therefore be an artefact of light exposure rather than a truly endogenous cycle.INDIVIDUAL DIFFERENCES AND REPETITIONS
Siffre himself admitted that his rhythms varied between studies. In 1972, he reported a 25-hour cycle; in 1999, after spending time in a Texas cave, he found his sleep–wake pattern sometimes extended to 48 hours. This inconsistency raises doubts about replicability and suggests that stress, boredom, and cognitive factors (rather than pure biology) may have influenced his behaviour.TECHNOLOGICAL AND CONTROL LIMITATIONS
Siffre’s studies were conducted before modern light measurement and hormonal testing were available. He could not measure melatonin or cortisol levels, relying only on behavioural markers (sleep and wake times). As a result, his data lack the physiological precision of later work by Czeisler and Duffy at Harvard, who used controlled environments, measured lux levels, and tracked metabolic markers.THEORETICAL VALUE AND MODERN RELEVANCE
Despite these flaws, Siffre’s work remains historically significant. It provided the first direct evidence that humans possess an endogenous circadian pacemaker independent of external cues. However, modern circadian neuroscience—incorporating controlled lux levels, hormonal assays, and genetic analysis—has refined and corrected his conclusions. Contemporary evidence shows that the human clock averages 24 hours and 11 minutes, not 25, and is continually stabilised by light, temperature, and social zeitgebers.
OTHER CAVE AND ISOLATION STUDIES
ASCHOFF & WEVER (1976)
Participants lived in underground bunkers free of time cues for several weeks. Most developed free-running rhythms of 25–27 hours, supporting Siffre’s findings. A minority showed extreme drift beyond 30 hours, suggesting individual differences in pacemaker stability.
FOLKARD ET AL. (1985)
Further support comes from Folkard et al. (1985), who studied twelve participants living in temporal isolation for three weeks. They were cut off from natural light and agreed to follow a strict schedule of sleeping from 11.45 p.m. to 7.45 a.m. Unbeknownst to them, the researchers gradually sped up the experimental clock so that what appeared to be a 24-hour day actually lasted 22 hours. All but one participant continued to follow the shortened schedule, suggesting that while humans have a strong internal rhythm, it can be modified by external time cues. However, participants were not completely isolated from artificial light, which was later found to act as a weak zeitgeber capable of resetting the internal clock.
WEVER’s “ISOLATION UNIT” STUDIES
Wever repeated multiple trials examining circadian rhythms in isolation. Participants showed stable but slightly long rhythms, with many drifting toward 25–26 hours. Notably, some participants exhibited two concurrent rhythms (a “split rhythm”), indicating that different biological systems may drift to varying rates without external synchronisation.
RECHTSCHAFFEN & MORE (ANIMAL ISOLATION AND PACEMAKER LESION STUDIES)
Animal studies where the SCN was damaged or removed showed complete loss of circadian rhythmicity. Rats without an SCN displayed random sleep–wake patterns that no longer followed a 24-hour cycle. When SCN tissue from another animal was transplanted, rhythmicity returned—proof that the SCN is the endogenous pacemaker controlling circadian timing.
OVERALL CONTRIBUTION OF ISOLATION STUDIES
Together, these studies show that:
• Biological rhythms persist without external cues, proving the existence of endogenous pacemakers.
• The natural length of the human circadian rhythm is slightly over 24 hours, often around 25 hours.
• Without zeitgebers such as light, rhythms drift, become unstable, and desynchronise from each other.
• Light is the key zeitgeber required to anchor internal rhythms to the 24-hour world.
• There are individual differences in pacemaker precision and sensitivity to cues.
• Age affects the stability and length of the free-running rhythm.
This body of research forms the backbone of the AQA Biological Rhythms topic and provides the strongest empirical support for the interaction between endogenous pacemakers and exogenous zeitgebers.
ANIMAL STUDIES (ANIMAL ISOLATION AND PACEMAKER LESION STUDIES)
MOORE AND EICHLER (1972) first identified the SCN in the hypothalamus as the brain’s master circadian pacemaker. Animal studies showed that SCN lesioning in rats abolished regular sleep–wake and hormonal cycles, confirming that the SCN is the central timing structure. The SCN receives light input via the retinohypothalamic tract, and this discovery established the first direct neural pathway linking environmental light to biological timing.
RECHTSCHAFFEN & MORE Animal studies where the SCN was damaged or removed showed complete loss of circadian rhythmicity. Rats without an SCN displayed random sleep–wake patterns that no longer followed a 24-hour cycle. When SCN tissue from another animal was transplanted, rhythmicity returned—proof that the SCN is the endogenous pacemaker controlling circadian timing.
RALPH ET AL. (1990) demonstrated that these rhythms could be transferred biologically. By transplanting SCN tissue from mutant hamsters (with 20-hour cycles) into normal hamsters, the recipients adopted the donor’s shortened rhythm. This provided robust evidence that circadian timing is endogenously generated, rather than merely a response to external cues.
KING AND TAKAHASHI (1991). Later, molecular neuroscience identified the genetic basis for these internal rhythms. King and Takahashi (1991) isolated the first clock gene in mice, named CLOCK, and subsequent work by Reppert and Weaver (2002) identified a network of genes (PER1, PER2, CRY, BMAL1) that produce self-sustaining 24-hour oscillations in almost every cell of the body. These findings confirmed that the circadian system is hierarchical: the SCN coordinates the rhythm, but each organ contains its own molecular clock that can drift out of sync when external cues change too rapidly.
CLIFFORD SAPER (2018) A related experiment by Clifford Saper (2018) at Beth Israel Deaconess Medical Centre used mice exposed to a 20-hour light–dark cycle. The animals exhibited disturbed metabolic function, further suggesting that circadian misalignment disrupts energy balance and hormonal rhythms. Saper’s work also identified links between circadian timing and aggression, showing that disruption affects not only metabolism but also behavioural regulation through altered brain circuits in the hypothalamus.
STUDIES ON THE BLIND
MILES ET AL (1997)
Further evidence for the biological basis of circadian rhythms comes from Miles et al. (1977), who investigated a blind man born without light perception. Despite exposure to social cues such as clocks and routines, his circadian rhythm remained locked at around 24.9 hours. He was forced to take stimulants in the morning and sedatives at night to align his sleep with the 24-hour day. This demonstrates that exogenous zeitgebers such as social cues alone cannot override endogenous biological timing mechanisms.
Subsequent studies confirm that many totally blind people experience non-24-hour sleep–wake disorder, where their biological night gradually moves through the 24-hour day. However, some partially sighted individuals can still entrain to light because they retain functioning melanopsin-containing retinal ganglion cells, which detect brightness even without visual perception. This shows that light detection, not sight itself, is critical for circadian regulation.
Overall, evidence from blindness supports the conclusion that light is the dominant environmental cue for human circadian rhythms and that loss of light input to the SCN causes the body’s internal clock to free-run independently of the external world.
SLEEP DISRUPTION STUDIES
CZEISLER ET AL. (1999) later reported neuroimaging and electrophysiological research showing how environmental disruption affects human brain activity. Czeisler et al. (1999) demonstrated that exposure to low-intensity artificial light (150–200 lux) can shift circadian phase and suppress melatonin, overturning Siffre’s assumption that dim light has no biological effect. Subsequent fMRI studies in the 2000s revealed that when the SCN is misaligned — as in shift work or jet lag — activity in the prefrontal cortex decreases, impairing decision-making and attention. At the same time, abnormal signalling between the SCN and amygdala increases emotional reactivity.
BUIJS ET AL. (2003. Neuroscience has also shown how circadian desynchrony affects stress and metabolism. Disruption of the SCN leads to dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, resulting in cortisol peaks at inappropriate times and heightened anxiety and cardiovascular strain (Buijs et al., 2003). Peripheral clocks in organs such as the liver and heart also fall out of phase, explaining why irregular shift work is associated with metabolic disorders, diabetes, and obesity (Hastings et al., 2007).
ARENDT (2010) and colleagues used molecular assays to show that after long-distance flights, the SCN adjusts to new time zones faster than peripheral tissues, leaving the body in temporary internal desynchronisation — the physiological basis of jet lag. The re-synchronisation of gene expression across these tissues can take several days, consistent with subjective recovery times reported by travellers.
JEAN DUFFY ET AL. (2018) Recent laboratory research provides strong modern evidence for how circadian disruption affects human physiology and long-term health. At Brigham and Women’s Hospital (BWH), Jeanne Duffy et al. (2018) conducted a controlled study in which volunteers lived for a month in windowless, soundproof rooms without any external time cues. Participants followed a 28-hour sleep–wake cycle, delaying sleep and meals by four hours each day while researchers measured glucose, insulin, and other metabolic markers. The findings showed that prolonged misalignment between internal circadian timing and external routines impaired glucose regulation and insulin sensitivity, mimicking early signs of type 2 diabetes. This supports epidemiological data showing that night-shift workers are more prone to weight gain, metabolic syndrome, and cardiovascular disease. The study demonstrated that even when total sleep time and calorie intake were controlled, shifting the timing of sleep and eating disrupted metabolism — confirming that when we eat and sleep, timing matters as much as quantity.
RESEARCH EVALUATION CIRCADIAN RHYTHMS
WHAT EVERYDAY APPLICATION COULD WE GAIN FROM THESE STUDIES?
DOES THE RESEARCH TELL US WHICH IS MOST IMPORTANT: ENDOGENOUS PACEMAKERS OR EXOGENOUS ZEITGEBERS?
RESEARCH SYNOPSIS
Taken together, research findings show a clear chronological progression
(1972) Identification of the SCN as the master clock.
(1990) Experimental proof that circadian timing is endogenous and transplantable.
(1991–2002) Discovery of molecular clock genes in the SCN and peripheral tissues.
(1999–2003) Demonstration that artificial light and shift work disrupt melatonin and cortical activity.
(2007–2010) Evidence linking circadian desynchrony to hormonal, metabolic, and cognitive dysfunction.
This body of evidence shows that circadian rhythms are hardwired into both neural and genetic systems. Deep within the brain, the suprachiasmatic nucleus (SCN) coordinates timing signals, while inside nearly every cell, clock genes such as CLOCK, BMAL1, PER1–3, and CRY1–2 create self-sustaining feedback loops. These genes encode proteins that oscillate in a roughly 24-hour cycle, regulating metabolic, hormonal, and behavioural rhythms. Remarkably, these molecular oscillations persist even in constant darkness or under stable conditions, proving they are endogenously generated rather than driven by the environment.
Although the circadian system can adapt gradually to seasonal or latitudinal changes, it remains biologically constrained. The human body is finely tuned to the Earth’s 24-hour rotation. It is easily destabilised by the rapid, irregular demands of modern life, such as shift work, jet lag, and exposure to artificial light.
UNIVERSALITY OF CIRCADIAN RHYTHMS
The fact that circadian rhythms exist in almost identical form across the living world is one of the strongest pieces of evidence that they are driven by powerful endogenous mechanisms rather than just external cues. FROM PLANTS TO HUMANS – THE SAME BASIC DESIGN
As early as 1729, Jean-Jacques de Mairan showed that mimosa plants continue to open and close their leaves on a ~24-hour cycle even in constant darkness – proving that an internal clock exists.
In 1971, Konopka & Benzer discovered the first clock gene (period) in fruit flies; mutants with a broken period gene had 19-hour, 28-hour, or no rhythms at all, even in regular light-dark cycles.
The same core molecular feedback loop (PER, CRY, CLOCK, BMAL1 proteins) is found in plants, fungi, insects, fish, mice, and humans.
MAMMALIAN SYSTEM – UNIVERSAL BUT HIERARCHICAL
In mammals, the suprachiasmatic nucleus (SCN) acts as the master pacemaker, but almost every cell in the body has its own peripheral clock running the same genetic loop. These tissue clocks are usually kept in perfect phase by the SCN. Still, they can also be shifted independently by feeding time, exercise, or temperature – exactly the mechanism seen in flies and plants.
WHY UNIVERSALITY MATTERS FOR THE ENDOGENOUS VS EXOGENOUS DEBATE
If circadian rhythms were mainly a passive response to external zeitgebers, we would expect huge differences between species and little need for conserved clock genes. Instead:
The molecular machinery is remarkably similar from algae to humans.
Rhythms persist (free-run) in constant conditions in every organism studied.
Disruption of the clock genes produces the same kinds of sleep, metabolic, and mood disorders in flies, mice, and humans.
CONCLUSION – A UNIVERSAL ENDOGENOUS DESIGN
The near-universal presence of the same genetic oscillator across the kingdoms of life demonstrates that circadian timing is a fundamental, evolved biological property rather than an environmental imposition. Exogenous zeitgebers are essential for synchronisation, but the core 24-hour rhythm is built into the organism itself. This universality underpins everything from plant agriculture to human chronotherapy. It explains why shift work and jet lag are so damaging – we are fighting a clock that has been ticking inside living things for hundreds of millions of years.
REAL-LIFE APPLICATION OF CIRCADIAN RHYTHMS: CHRONOPHARMACOLOGY
Circadian rhythms have practical consequences for health and behaviour. Many physiological processes—such as metabolism, hormone release, alertness and enzyme activity—rise and fall across the 24-hour cycle. As a result, some medications work better at certain times of day (a field known as chronopharmacology). For example, blood-pressure drugs are often more effective when taken in the evening because that is when nighttime blood pressure begins to rise. At the same time, asthma medication may be more beneficial later in the day when lung function naturally dips. Even pain sensitivity, reaction time and cognitive performance follow daily circadian patterns. The timing of drug dosing has been studied for anticancer, cardiovascular, respiratory, anti-ulcer, and anti-epileptic drugs (Baraldo, 2008).
PLEASE NOTE
PHARMACOKINETICS
What the body does to the drug (absorbs it, spreads it around, breaks it down, gets rid of it).
That’s it. It’s just the science of how drugs move inside you.
Circadian rhythms can change how fast or slow these things happen during the day, but the word itself doesn’t mean “choosing the best time to take the medicine”.
CHRONOPHARMACOLOGY
Giving the drug at the perfect time of day on purpose because you know the body clock makes it work better (or have fewer side effects) at that time.
Examples: blood-pressure tablets at night, asthma inhalers in the evening, some cancer drugs at specific hours. WHICH ONE IS THE REAL-LIFE
APPLICATION OF CIRCADIAN RHYTHMS?
Chronopharmacology.
WHY TEXTBOOKS AND TEACHERS KEEP SAYING “PHARMACOKINETICS” INSTEAD
Circadian rhythms change things like liver activity and blood flow during the day → these changes do affect pharmacokinetics → teachers and books therefore say “circadian rhythms affect pharmacokinetics” and stop there.
They never take the next step and say the actual application is choosing the best time to give the drug.
So the word “pharmacokinetics” is misused as the whole answer when the question is about practical use (i.e., timing the dose = chronopharmacology).
BOTTOM LINE FOR YOUR EXAM
If the question says “application”, “example”, or “importance” of circadian rhythms in medicine, describe the timing of the drug (e.g., blood-pressure drugs at night) and, if you want to be precise, call it chronopharmacology. Do not just write “pharmacokinetics”.
SHIFT WORK AND JET LAG: DISRUPTION BY EXOGENOUS ZEITGEBERS
Exogenous zeitgebers are the external cues (light, meal times, social schedules, temperature) that usually keep our endogenous circadian rhythm locked to the 24-hour day. When these cues become inconsistent or change too quickly, the master clock in the suprachiasmatic nucleus (SCN) and the peripheral clocks in organs such as the liver and heart lose synchrony. Rotating shift patterns are particularly damaging because the body never has enough days to fully re-entrain before the next switch. Permanent night shifts, although still unnatural, allow partial re-entrainment when light and meals are kept consistent. Jet lag works the same way on a shorter timescale: rapid travel across time zones abruptly disrupts the light-dark cycle, and eastward travel is harder because it requires a phase advance (shortening the day). In contrast, westward travel only requires the easier phase delay.. For this reason, unless constantly travelling across time zones, jet lag is less disruptive than shift work.
HEALTH CONSEQUENCES OF MISALIGNMENT
The result is internal desynchronisation. Performance crashes at the circadian trough around 4–6 a.m., producing the well-documented rise in accidents at the end of night shifts (Boivin et al., 1996). Long-term effects include chronically low melatonin, high cortisol, obesity, type 2 diabetes, and a threefold increase in cardiovascular disease (Knutsson, 2003). Shift work is also linked to higher rates of breast cancer, gastrointestinal disorders and mood disturbance. As Russell Foster (2013) emphasises, persistent disruption of the sleep-wake cycle impairs memory consolidation, weakens immune function, destabilises mood and significantly raises the risk of dementia and early death.
PRACTICAL APPLICATIONS: USING EXOGENOUS ZEITGEBERS TO RE-ALIGN THE SYSTEM
Because conflicting zeitgebers cause the problem, we can deliberately control the same cues to restore alignment:
Deliver bright light (>1000 lux) immediately after waking, whatever time that is.
Create complete darkness, cool temperature (16–18 °C) and quiet during intended sleep hours, even if that sleep occurs in daylight.
Keep meal times fixed to entrain the liver’s food-entrainable oscillator.
Avoid caffeine, alcohol and blue light in the biological evening.
Use blackout blinds, eye masks and earplugs to block unwanted light and noise.
For jet lag, gradually shifting light exposure and meal times in the days before travel dramatically shortens recovery time.
WIDER SOCIETAL BENEFITS
When individuals apply these evidence-based adjustments, accident rates in healthcare, transport and industry fall, absenteeism decreases, and the long-term burden of cardiovascular disease, diabetes, cancer and mental health problems is reduced. The NHS benefits directly from lower demand for treatment of these largely preventable conditions, while employers gain a healthier, more reliable workforce. Understanding exogenous zeitgebers, therefore, provides low-cost, high-impact solutions to some of the biggest health challenges created by modern 24-hour society.
INDIVIDUAL DIFFERENCES IN CIRCADIAN RHYTHMS
The idea that everyone’s body clock runs in the same way is a myth. Even when people live in the same time zone and are exposed to identical light-dark cycles, huge differences appear in preferred sleep-wake times, peak alertness, and overall circadian phase.
MORNINGNESS VS EVENINGNESS (CHRONOTYPES)
Duffy et al. (2001) showed that “morning people” (larks) naturally wake around 6 a.m., feel most alert in the early part of the day, and prefer to sleep by 10 p.m. “Evening people” (owls), in contrast, struggle to wake before 9–10 a.m., reach peak performance in the late afternoon/evening, and do not feel sleepy until 12–2 a.m. or later. These preferences are remarkably stable across the lifespan and are seen worldwide.
WHY THIS MATTERS FOR EXOGENOUS ZEITGEBERS
Both larks and owls experience the same sunrise, sunset, and artificial light patterns, yet their internal clocks are offset by several hours. This proves that exogenous zeitgebers such as light, while powerful, are not the whole story. The same light exposure advances the clock of a morning person but may have a much weaker or delayed effect on an evening person. In other words, the strength and timing of responses to external cues vary biologically across individuals.
BIOLOGICAL AND GENETIC BASIS
Chronotype is highly heritable (around 40–50 %). Twin studies and genome-wide association studies have identified multiple “clock gene” variants (e.g., PER3, CLOCK) that lengthen or shorten the intrinsic period of the circadian cycle. Morning types tend to have a slightly shorter intrinsic period (<24 h), making it easier for them to phase-advance in response to morning light. In contrast, evening types often have a longer intrinsic period (>24 h), which resists early waking.
DEVELOPMENTAL CHANGES: AGE DIFFERENCES IN CIRCADIAN RHYTHMS
A common student critique of the role of exogenous zeitgebers runs like this:
“If light and social cues are so powerful, why do sleep patterns change so dramatically across the lifespan? Babies sleep around the clock, teenagers won’t get up before lunchtime, and grandparents are awake at 5 a.m. Doesn’t this prove that external zeitgebers override the internal clock?”At first glance, it looks convincing. In reality, these age-related shifts are driven by biological changes in the endogenous circadian system itself, not by external cues winning the battle.
DEVELOPMENTAL CHANGES – BIOLOGY, NOT ENVIRONMENT
Newborns have no stable 24-hour rhythm at birth. Melatonin only becomes rhythmic around 3 months, and cortisol between 2–9 months (NCBI, 2023). Until then, sleep is scattered because the endogenous pacemaker is still maturing.
Adolescence triggers a programmed 2–3 hour delay in melatonin release – the biological cause of the famous teenage “sleep phase delay”. It happens in every culture, even when school start times and light exposure are identical to those of adults.
In older age, melatonin production drops, circadian amplitude weakens, and sleep fragments, pushing wake times earlier.
These are endogenous developmental changes, not evidence that external zeitgebers are taking over.
CONCLUSION
These findings show that circadian rhythms are biologically flexible and individually unique. Development, genetics, and lifestyle interact to produce distinct sleep–wake patterns, highlighting that the human circadian system is an adaptive, not uniform, mechanism.
REAL-WORLD APPLICATIONS — ARTIFICIAL LIGHT AND TECHNOLOGY
Modern environments expose people to artificial light far beyond what the brain evolved to handle. The human internal clock (regulated by the suprachiasmatic nucleus, or SCN) depends on natural light as its main zeitgeber—but artificial lighting, especially from screens and LED bulbs, interferes with this system.
Light intensity, measured in lux, is key. Natural daylight can exceed 10,000–100,000 lux, while indoor lighting averages only 150–500 lux, and most screens emit around 30–150 lux. Although weaker overall, LED and phone screens emit a high proportion of blue light (≈480 nm), which stimulates melanopsin-containing retinal cells that send light signals to the SCN. This tricks the brain into thinking it is still daytime, suppressing melatonin and delaying sleep onset.
Evidence from Czeisler et al. (1999) demonstrated that even dim light (150–200 lux) can shift circadian phase, showing that ordinary indoor illumination is enough to disrupt the body clock. Subsequent cognitive neuroscience studies using fMRI have shown that evening light exposure alters neural activity in the prefrontal cortex and hippocampus, impairing memory consolidation, attention, and emotional regulation. Long-term disruption is associated with insomnia, depression, and metabolic disorders.
These findings have clear real-world implications. They explain why using phones or laptops before bed can delay sleep and reduce rest quality, and why exposure to bright screens in the evening contributes to widespread sleep disturbance in modern societies. Understanding the relationship between lux levels, wavelength, and SCN signalling has informed practical solutions such as blue-light filters, night-shift lighting, and circadian-based light therapy to restore alignment between the internal clock and the natural light–dark cycle.
CULTURAL AND GEOGRAPHICAL VARIATION IN CIRCADIAN RHYTHMS: WHY LIGHT IS NOT ALWAYS THE STRONGEST ZEITGEBER
In almost every discussion of circadian rhythms, light is treated as the overwhelmingly dominant exogenous zeitgeber. This emphasis is understandable – light is powerful and easy to study – but it quietly assumes the same rule applies to every human on Earth. When we look at people living in extreme environments, that assumption collapses.
EQUATORIAL REGIONS – TEMPERATURE EASILY OVERPOWERS LIGHT
At the equator, the day is 12 hours long every single day of the year. Light-dark variation is tiny and gives almost no daily timing information. Instead, the daily temperature cycle takes over. Core body temperature drops in the early afternoon, exactly when external heat peaks; alertness crashes, and the siesta is born. From southern Spain and Italy to Mexico, Nigeria, the Middle East and Southeast Asia, afternoon rest is universal for the same biological reason. Buhr et al. (2010) showed that temperature swings of less than 1 °C are enough to entrain peripheral clocks even when light is constant. When the environment becomes hotter than the body can comfortably handle, temperature becomes a stronger zeitgeber than light.
HIGH-LATITUDE EXTREMES – NATURAL LIGHT VANISHES FOR MONTHS
Above the Arctic Circle (northern Norway, Sweden, Finland, Iceland, Alaska, Siberia) the sun disappears completely in winter and never sets in summer. For half the year, there is no daily light-dark cycle at all. Pre-industrial populations did not force a single consolidated night's sleep. They used biphasic or polyphasic patterns and relied on social zeitgebers (fixed meals, prayer, communal storytelling) and behavioural cues tied to twilight. Even today, the total absence of natural light-dark contrast still causes widespread winter insomnia and summer sleep disruption unless people deliberately create artificial cues (dawn simulators in winter, blackout curtains in summer).
EVOLUTIONARY REASON: THE SCN IS FLEXIBLE, NOT RIGID
A completely fixed, light-only clock would have been lethal for early humans. The group always needed some individuals awake at night to tend the fire, guard the camp, nurse infants, care for the sick, or watch for predators and dawn hunting opportunities. Natural selection, therefore, favoured a circadian system that could prioritise whichever zeitgeber was most reliable and survival-critical in the local environment: light on the open savannah, temperature near the equator, social and activity cues in forests or polar regions. Studies of present-day hunter-gatherers and historical records (Ekirch, 2005; Yetish et al., 2015) confirm that segmented sleep with a natural wakeful period around 2–3 a.m. was the ancestral pattern – ideal for quiet vigilance when danger was highest.
MODERN EVOLUTIONARY MISMATCH
The same flexibility that allowed us to colonise every habitat now works against us. Artificial electric light (only ~140 years old), blue-rich screens, transatlantic flights, night shifts and 24-hour society give us weak, conflicting zeitgebers that our biology never evolved to handle. The SCN still expects the strong, clear signals our ancestors had; instead, we live in perpetual mild desynchronisation, paying for it with rising rates of obesity, diabetes, heart disease, cancer and mood disorders.
CONCLUSION
Human circadian rhythms are not universally light-driven. The system evolved to detect and follow whichever zeitgeber best predicts survival conditions in a given habitat. This built-in flexibility explains equatorial siestas, high-latitude segmented sleep, and why modern artificial environments are making so many of us ill. It removes the ethnocentric bias that treats light as the only “proper” zeitgeber and shows the human circadian system for what it really is: a highly adaptive, interactionist mechanism shaped by both nature and nurture.
REDUCTIONISM: BIOLOGICAL RHYTHMS CANNOT FULLY EXPLAIN SLEEP BEHAVIOUR
Although the SCN sets the timing of the sleep–wake rhythm, it does not control sleep on its own. Other neural systems are essential for initiating and maintaining sleep. The VLPO in the hypothalamus triggers sleep by releasing GABA to inhibit wake-promoting neurons. At the same time, the ARAS in the brainstem keeps the brain alert through neurotransmitters such as acetylcholine and norepinephrine. Orexin pathways stabilise wakefulness, and loss of orexin cells causes narcolepsy. These systems show that the SCN only determines when sleep should occur; it does not determine whether sleep actually happens.
This means that explanations based solely on circadian pacemakers are biologically reductionist, because they overlook the broader network of mechanisms required for regular sleep. A complete account of sleep behaviour must therefore include both the timing provided by biological rhythms and the neural systems that control sleep states themselves.
INFRADIAN RHYTHMS
Another crucial biological rhythm is the infradian rhythm. Infradian rhythms last longer than 24 hours and can be weekly, monthly or annually.
Two examples of these are:
The Menstrual Cycle
Biannual Oestrus in some mammals
THE ENDOGENOUS PACEMAKER FOR THE MENSTRUAL CYCLE
THE MENSTRUAL CYCLE
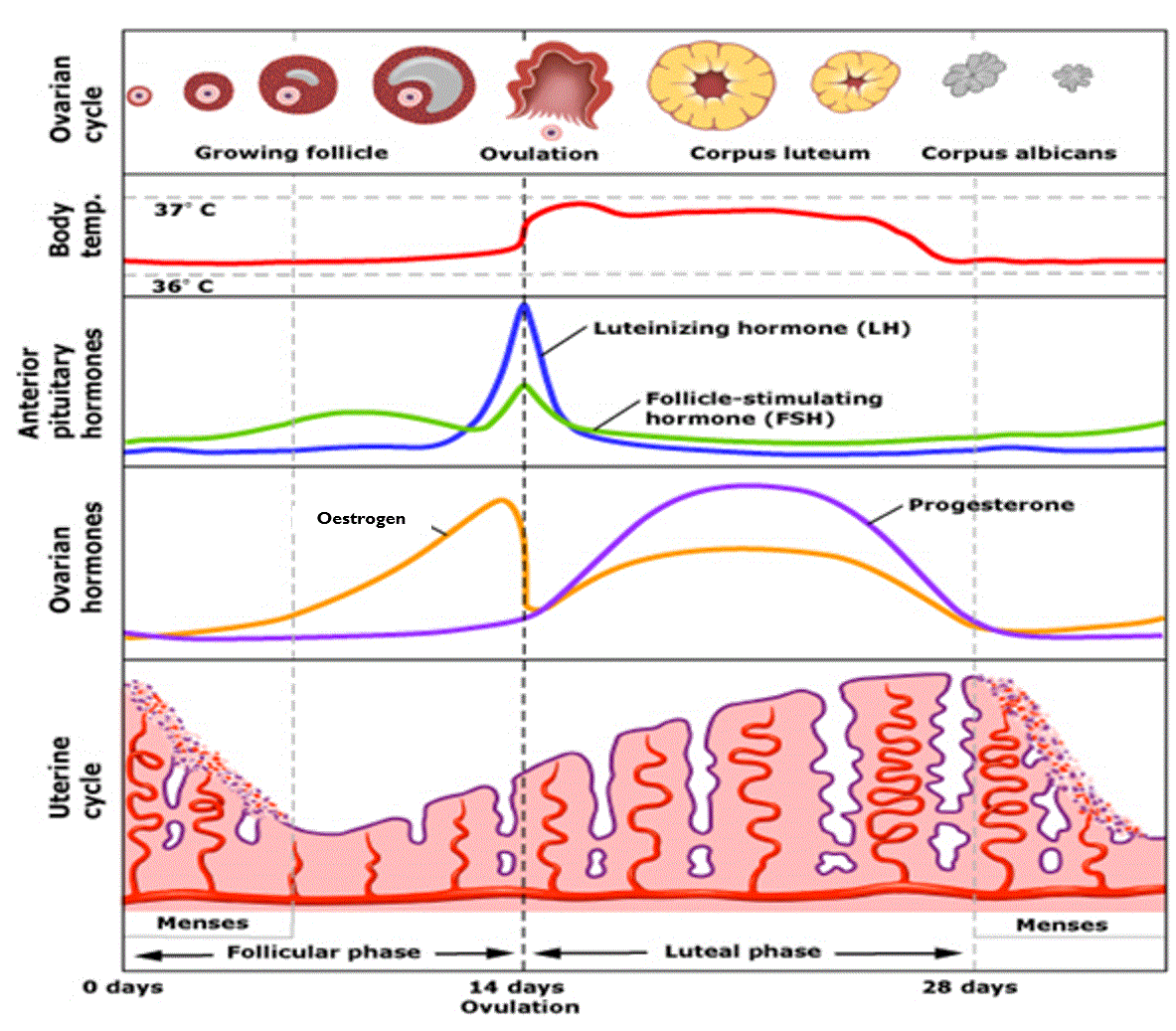
The menstrual cycle is a clear example of an infradian rhythm because it follows a repeating biological pattern that lasts longer than twenty-four hours, typically around twenty-eight days. It consists of a predictable sequence in which the body prepares for the possibility of pregnancy. The cycle begins with menstruation, when the uterine lining is shed. After this, hormone levels rise and the lining begins to regenerate. Midway through the cycle, a surge in luteinising hormone triggers ovulation. If fertilisation does not occur, levels of oestrogen and progesterone fall, causing the lining to break down and initiating the next cycle. Although the exact length varies between individuals, the overall pattern is regular and cyclical, which is why it is classified as an infradian rhythm.
An endogenous pacemaker governs this rhythm: the hypothalamic–pituitary–ovarian (HPO) axis. At the centre of this system is the hypothalamus, which sets the pace of the cycle by releasing pulses of GnRH. These pulses instruct the pituitary gland when to release FSH and LH, which control follicle development and ovulation. In turn, the ovaries produce oestrogen and progesterone, and these hormones feed back to the hypothalamus and pituitary to regulate the next stage of the cycle. This closed feedback loop allows the menstrual rhythm to continue in a coordinated monthly pattern, even without external cues.
Across the cycle, these hormonal fluctuations can also affect mood, energy and behaviour. Rising oestrogen in the first half of the cycle is associated with increased motivation and cognitive clarity. The LH surge around ovulation often coincides with heightened vitality and sexual interest. Later in the cycle, falling progesterone can contribute to premenstrual symptoms such as irritability or low mood. These effects occur because the hormones that regulate the cycle also influence brain function. They do not alter the timing of the rhythm itself but show how infradian processes can produce psychological and behavioural changes.
Taken together, the menstrual cycle demonstrates how an infradian rhythm is internally organised by a biological pacemaker. A sequence of hormonal signals generates a stable monthly pattern and produces characteristic physiological and psychological changes as the cycle progresses
IN SUMMARY
The menstrual cycle is an infradian rhythm because it repeats roughly every 28 days. It is controlled internally by the HPO axis, which releases hormones in a fixed order to prepare the body for pregnancy. These hormones also affect mood and energy, with oestrogen lifting mood earlier in the cycle and falling progesterone causing premenstrual symptoms later on. It is a clear example of an internal biological rhythm that repeats monthly
EXOGENOUS ZEITGEBERS OF THE MENSTRUAL CYCLE
The menstrual cycle is an infradian rhythm generated internally by the hypothalamic–pituitary–ovarian system, but it does not operate in a vacuum. Infradian reproductive rhythms evolved to adapt to the broader environmental landscape because successful reproduction depends on long-term conditions rather than moment-to-moment cues. For this reason, external factors act as exogenous zeitgebers—not by “resetting” the cycle as light resets the SCN, but by modulating the phase, stability, and regularity of the rhythm in response to the outside world. These zeitgebers operate over weeks or months, reflecting the adaptive need to align fertility and reproduction with environmental safety, resource availability and social context.
SOCIAL AND PHEROMONAL CUES
Social context is a potential zeitgeber because reproductive timing historically benefited from cooperative breeding, resource sharing and synchronised childrearing. Even though evidence is mixed, the idea is psychologically coherent:
Group living creates shared environmental patterns that influence behaviour and emotional states.
Exposure to others’ pheromonal or behavioural cues may provide information about social structure, safety and coordination.
Infradian rhythms evolved under conditions where synchrony increased offspring survival—not necessarily through perfect alignment, but through shared labour and protection.
Thus, social cues act as potential zeitgebers not because they “trigger ovulation,” but because they serve as social-environmental information that may interact with monthly timing.
RESEARCH ON SOCIAL / PHEROMONAL CUES AS A ZEITGEBER
SUPPORTING STUDIES
McClintock (1971)
Observed synchronisation of menstrual onsets among 135 dormitory students. Interpreted as menstrual synchrony driven by social/pheromonal cues.
Russell, Switz & Thompson (1980)
Applied underarm sweat from donors to recipients’ upper lips. Recipients showed small shifts in cycle timing toward the donor’s cycle phase.
Weller & Weller (1992, 1993, 1997)
In multiple cohabiting groups (mother–daughter pairs, kibbutz communities, lesbian couples), menstrual onsets moved somewhat closer together over time.
Cutler, Friedman & McCoy (1998) – MISSING ON MOST A-LEVEL PAGES
Found that exposure to synthetic putative human pheromones increased the likelihood of regular cycles and ovulation in women using pheromone-infused perfume. Suggested external olfactory cues can influence cycle regularity.
Preti et al. (2003) – ALSO MISSING FROM SCHOOL SITES
Showed that compounds from women’s axillary secretions applied to other women produced modest timing shifts in LH surge patterns, suggesting odour-mediated cycle influence.
Stern & McClintock (1998)
Daily application of axillary secretions (pads worn under arms) from donors at different cycle phases shifted cycle timing in 68 per cent of recipients toward the donor phase.
CRITICAL AND DISCONFIRMING STUDIES
Wilson (1991)
Repeated McClintock’s dormitory study with better controls and found no synchrony beyond chance expectation.
Strassmann (1997, 1999)
A long-term prospective study of Dogon women (natural fertility) found no menstrual synchrony at all, even under ideal conditions for detecting it.
Yang & Schank (2006)
A large sample (N = 186) in Chinese dormitories showed no synchrony and demonstrated how “synchrony” appears statistically by chance when cycle lengths vary naturally.
Schank (2006)
A comprehensive review concluded that evidence for human menstrual synchrony is weak, inconsistent, and methodologically flawed, and that pheromonal influence is minimal at best.
Treloar et al. (1967–1970 longitudinal cohort) – RARELY TAUGHT, BUT IMPORTANT
Showed huge natural variability in cycle length across months and individuals, meaning synchrony can appear by coincidence in groups of women. Undercuts claims of pheromone effects.
Jern & Haukka (2015) – CONTEMPORARY STUDY
A large dataset of couples and cohabiting women found no synchrony, challenging the assumption that close contact reliably shifts menstrual timing.
STRESS AS A ZEITGEBER
Stress affects the menstrual cycle because chronic sympathetic arousal communicates an extended period of threat, instability or unpredictability. Unlike circadian rhythms, which depend on a single, non-negotiable environmental cue (light), the menstrual cycle integrates ecological cues. Sustained stress signals that the external world may not support pregnancy or infant survival.
This happens for several interconnected psychological and biological reasons:
Sympathetic dominance prioritises mobilisation of resources for vigilance, defence and action. Reproductive timing is deprioritised because pregnancy requires sustained internal stability rather than acute reactivity.
HPA-axis activation creates a long-term profile of arousal that competes with reproductive rhythms. This does not simply “switch off” the cycle; it introduces noise and instability into the pattern, shifting the cycle length, the onset of ovulation, and hormonal coherence.
Environmental inference: from an evolutionary psychology perspective, stress serves as informational input about danger, scarcity or disruption within the habitat. Reproductive timing adjusts because these contexts reduce the likelihood that offspring will survive.
Stress (umbrella zeitgeber)
– includes sympathetic arousal
– chronic unpredictability
– threat cues
– instability
– significant environmental changes (relocation, lifestyle disruption, dramatic shifts in routine)
– prolonged cognitive/emotional load
– extended physiological demand
Thus, stress functions as a contextual zeitgeber—a long-term environmental signal integrated into the infradian rhythm's pacing.
This is deep, rhythm-specific, and qualitatively different from circadian entrainment.
RESEARCH ON STRESS AS A ZEITGEBER
STRESS FROM WAR, DISASTER, AND EXTREME EVENTS
Grof & Schreiber (1976) – Earthquake stress and menstrual disturbance. After the 1971 San Fernando earthquake, women living in the affected region showed significantly higher rates of cycle length changes, delayed menstruation, and amenorrhoea compared with unaffected populations.
Largillier, 1983 – Lebanese Civil War study: Women exposed to chronic wartime stress displayed increased rates of oligomenorrhoea (infrequent menstruation) and cycle irregularity compared to those outside the conflict zone.
Morris & Udry (1970) – Bereavement: Women's cycles became irregular following the death of a close family member, with disrupted ovulation patterns for several months.
Hui et al. (2014) – Wenchuan earthquake: Adolescent girls in the earthquake zone reported increased menstrual irregularity for several months post-disaster compared with controls in neighbouring regions.
Implication: major environmental upheaval influences menstrual timing at the population level.
EXAM STRESS AND CHRONIC ACADEMIC PRESSURE
Harlow et al. (1996) found that university students showed longer, shorter, or skipped cycles during sustained academic stress (exam terms), not during isolated stressful events.
Nillni et al. (2011) Large sample of college students: high perceived stress was associated with cycle irregularity and variable cycle length, independent of lifestyle factors.
Implication: Psychological stress functions as a contextual zeitgeber over weeks to months.
HPA–HPG AXIS INTERACTION (MECHANISTIC EVIDENCE)
Berga & Loucks (2006) – Functional hypothalamic amenorrhoea: Women under chronic stress show elevated cortisol and significantly reduced GnRH pulsatility, disrupting ovulation—implication: chronic HPA activation interferes with reproductive timing mechanisms.
Sanders & Bruce (1997) found that stress hormones suppress luteinising hormone (LH) pulses, directly altering the rhythm of the menstrual cycle.
Implication: stress alters timing signals at the endocrine pacemaker level.Chrousos & Gold (1992) – HPA hyperactivation and reproductive suppression: Reviews evidence that prolonged cortisol elevation down-regulates the reproductive axis, delaying or preventing ovulation. Implication: the stress system and reproductive system compete for homeostatic priority.
CHALLENGING EVIDENCE
Harlow et al. (1999): Some women with high stress scores showed no significant cycle disruption, indicating individual differences in stress resilience.
Implication: stress is a probabilistic zeitgeber, not a deterministic one.Treloar et al. (1967–1970, 30-year longitudinal dataset): A large sample showed that menstrual variability naturally increases with age and across individuals, independent of stress. Implication: Cycle variability is partly intrinsic; external stress does not explain all patterns.
Barbieri (2001): Acute stress has minimal effect unless it becomes chronic or sustained; the menstrual system responds to persistent environmental conditions rather than momentary fluctuations—implication: infradian rhythms require long, stable ecological signals.
SUMMARY
Strong evidence (natural disasters, war, chronic academic stress) shows long-term stress alters menstrual timing.
Endocrine evidence shows HPA activation interferes with reproductive rhythm generation.
Individual differences mean not all women respond the same way.
Cycle variability is partly natural, so stress effects must be interpreted against baseline variation.
The menstrual cycle is influenced by environmental context, not precision “time cues” like circadian rhythms.
LACK OF FOOD AVAILABILITY AS AN EXOGENOUS ZEITGEBER EXPLANATION
Energy availability acts as a zeitgeber because it signals the environmental capacity to sustain pregnancy and childcare. The menstrual cycle evolved under conditions where food scarcity signalled ecological risk. Fertility is not simply “turned off”; rather, the monthly rhythm recalibrates in response to sustained energetic conditions.
Detailed psychological principles involved:
Environmental energetics: low or inconsistent food intake is interpreted as evidence that the habitat cannot reliably support gestation or infant feeding.
Long-term pattern detection: infradian rhythms respond to aggregate environmental patterns rather than isolated events. Weeks of reduced intake are read as a sustained environmental cue, prompting the system to adjust its pacing.
Adaptive reproductive strategy: reproduction is delayed until cues indicate that conditions have returned to a stable range.
This is why nutrition is not just a “biological factor” but a slow-timescale environmental cue that acts as a zeitgeber for the infradian rhythm.
LACK OF FOOD AVAILABILITY AS AN EXOGENOUS ZEITGEBER RESEARCH
FRISCH & McARTHUR (1974): Menarche is delayed in girls with low body fat; athletes and malnourished populations show a later onset of menstruation.
Relevance: Demonstrates that long-term energy availability regulates infradian reproductive timing.
Key point: The menstrual cycle responds to energetic conditions, not to fixed clock time.WARNOCK & REMSBURG (1984) – Weight loss and cycle length: Women who experienced significant, sustained weight loss showed longer cycle lengths and more anovulatory cycles.
Relevance: Reduced energy availability directly alters the rhythm.
Interpretation: Energy availability acts as a contextual zeitgeber signalling environmental conditions.LOUGHRAN et al. (1987) – High-performance athletes: Endurance athletes showed delayed ovulation, luteal phase defects and irregular cycles, especially during periods of intense training with high energy expenditure.
Relevance: Total energy balance, not just diet, is the critical environmental cue.BULLEN et al. (1985) – Negative energy balance. Short-term calorie restriction combined with intense exercise caused immediate suppression of ovulation in previously normal women.
Relevance: Demonstrates that the reproductive rhythm integrates sustained energy signals.DE SOUZA et al. (1998, 2003) – Exercise-induced menstrual disruption: Athletes with chronically low energy availability showed functional hypothalamic amenorrhoea, delayed ovulation and irregular cycles; restoring energy balance corrected the rhythm.
Relevance: Strong evidence that energy acts as an external environmental cue feeding into the infradian rhythm.LOUDON et al. (1998) – Ecological and evolutionary evidence: In subsistence and hunter–gatherer populations, seasonal food availability predicts seasonal conception and ovulation patterns.
Relevance: Natural fertility populations show that energy availability entrains both infradian and circannual reproductive rhythms.WARREN et al. (1992) – Energy availability vs psychological stress: Energy deficit, more than psychological stress, predicted menstrual disturbance in ballet dancers.
Relevance: Distinguishes energy as a direct environmental zeitgeber rather than just a correlate of stress.STEIN & SUSSMAN (2015) – Low energy availability threshold: Identified energy availability thresholds below which normal ovarian function cannot be sustained.
Relevance: Quantifies energy availability as a regulatory external cue influencing cycle regularity.
CHALLENGING / QUALIFYING EVIDENCE
NOT ALL WOMEN RESPOND IDENTICALLY: Some women maintain regular cycles even with substantial energy deficit (e.g., high-performing athletes), suggesting individual thresholds for sensitivity.
MULTI-FACTORIALITY: Studies on athletes often involve combined stressors (training load, psychological stress, lifestyle), making it difficult to isolate the nutritional cue alone.
VARIABILITY IN NATURAL FERTILITY POPULATIONS: Some groups show robust fertility despite variable food intake, suggesting that cultural and biological buffers modulate the effect.
SUMMMARY
There is robust evidence that energy availability acts as an exogenous zeitgeber influencing the menstrual cycle.
The menstrual rhythm responds to sustained environmental conditions rather than single cues.
Nutritional and energetic signals operate on infradian timescales, aligning reproductive timing with long-term environmental suitability.
Restoring energy availability can restore the rhythm, showing the cue is reversible and external.
EVALUATION OF RESEARCH ON INFRADIAN RHYTHMS
GENERAL METHODOLOGICAL WEAKNESSES IN MENSTRUAL RESEARCH
Much research on menstrual rhythms—whether examining stress, lifestyle factors or synchrony—shares broad methodological limitations. Studies commonly use:
Small, unrepresentative samples (mostly students) can’t generalise. Because of this variability, generalising from small or short-term studies is problematic, and random drift across cycles explains why “synchrony” appears in small groups.
Menstrual cycles vary substantially across individuals (commonly 21–35 days) and across the lifespan. Stress, illness and energy availability also affect timing.
Inconsistent definitions of cycle length
Short observation periods that do not capture natural drift
Inadequate control of confounders (stress, sleep, illness, weight changes, exercise).
Self-reports – Retrospective data: Most of the research requires women to self-report the onset of their cycle. This means we can’t be entirely sure whether what is reported is the truth.
Validity: One weakness is that research such as this is difficult to establish high internal validity. Russell et al. (1980) found that female menstrual cycles synchronised with those of other females through odour exposure.. However, other variables, such as diet, exercise, and stress levels, are not controlled. This would make it difficult to assume that the synchronisation occurred with the odour exposure..
These weaknesses reduce the reliability of findings in almost all infradian research, not only synchrony studies.
SPECIFIC PROBLEMS WITH SYNCHRONY RESEARCH
Research on social and pheromonal cues shows mixed results. Early studies reported cycle convergence among cohabiting women, but later analyses point to statistical artefacts and inconsistent replication. Current evidence suggests that social context may produce small, unstable timing shifts, but it is neither a strong nor a reliable zeitgeber. Its value is mainly conceptual: infradian rhythms may be somewhat sensitive to social environments, but this influence is weaker than that of stress and energy availability.
Synchrony research has additional methodological flaws:
• high probability of random synchrony
• statistical artefacts producing false convergence
• heavy reliance on inaccurate self-report
• failure to control natural variation in cycle length
• failure to control major physiological influences (stress, energy availability, illness)
When these issues are corrected, synchrony effects disappear. The most consistent conclusion is that menstrual synchrony is not a genuine biological phenomenon, but a statistical illusion.
EVOLUTIONARY SYNCHRONY EXPLANATIONS LACK EVIDENCE
If synchrony is not absolute, pheromonal explanations and evolutionary synchrony theories have no scientific footing
Evolutionary accounts proposing that synchrony improved group cohesion, shared childcare or breastfeeding are:
• speculative
• unfalsifiable
• unsupported by empirical findings
• logically weak (synchrony could increase competition for mates and resources)
Since synchrony itself lacks empirical support, evolutionary reasoning built on it has no scientific value.
STRESS – SUMMARY OF RESEARCH CONCLUSIONS
Research consistently shows that sustained stress can alter the timing of menstruation. Studies involving war exposure, natural disasters, chronic academic pressure, bereavement and significant life disruption all report higher rates of delayed cycles, irregularity and anovulation. These findings converge on the same point: the menstrual rhythm is sensitive to long-term environmental stability. Stress acts as a zeitgeber because chronic sympathetic and HPA activation signals that conditions are unsafe or unpredictable, and reproductive timing adjusts accordingly. This includes large-scale environmental changes such as relocation, shift-work or disruption of routine; these all operate through the same stress pathway and therefore belong under the same heading. Across populations, the pattern is clear, although individual responses vary.
NUTRITION / ENERGY AVAILABILITY – SUMMARY OF RESEARCH CONCLUSIONS
Evidence from athletes, controlled diet studies, weight-change cohorts and ecological populations shows that energy availability is one of the strongest external influences on menstrual timing. Low or inconsistent energy intake, high expenditure or seasonal shifts in food availability all correspond with delayed ovulation, longer cycles or temporary suppression of the rhythm. Restoring energy availability reliably restores regularity. Research across human and natural-fertility populations indicates that the menstrual cycle tracks long-term energetic conditions, making nutrition a central exogenous cue for infradian regulation.
CONCLUSION
The menstrual cycle is best understood as an endogenously generated infradian rhythm governed by the HPO axis.
However, it is strongly modifiable by exogenous factors such as stress, illness, and energy availability.
Claims that exogenous pheromonal cues synchronise cycles lack methodological support.
Likewise, evolutionary synchrony theories are speculative and unsupported.
Overall, the most scientifically robust explanation is that menstruation is an internal hormonal rhythm that interacts with powerful, non-social external conditions, not a rhythm shaped by pheromones or group synchrony.
SUMMING UP: ENDOGENOUS PACEMAKER AND EXOGENOUS INFLUENCES
Ultimately the menstrual cycle is controlled by an endogenous pacemaker: the hypothalamic–pituitary–ovarian (HPO) axis. This internal hormonal system generates the infradian rhythm even in the absence of external social input.
However, the cycle is highly sensitive to exogenous influences. These include:
• Chronic energy deficit
• Dieting, starvation (rapid weight change), Anorexia Nervosa, and poor nutrition
• Extreme exercise
• Illness
• Sympathetic arousal from stress, eg anything that causes the fight or flight response,
These are exogenous disruptors that act on the body by altering hormone production, thereby modifying, delaying, or suppressing the cycle.
A clear example is amenorrhoea in anorexia nervosa: starvation and very low body fat (both exogenous states) lead the body to suppress ovulation and menstruation adaptively. The mechanism is biological, but the trigger is exogenous—precisely as with the SCN responding biologically to light, which remains an exogenous zeitgeber.
MENSTRUATION DISRUPTION AND EVOLUTION
The menstrual cycle is an infradian reproductive rhythm, not a survival rhythm. If it pauses or shifts, the individual remains physiologically stable. Because pregnancy requires long-term safety, energy and stability, reproductive rhythms evolved to adjust their timing when the external environment signals threat, instability or low resources. This includes chronic stress, significant lifestyle disruption and poor energy availability—signals that the wider conditions are not suitable for supporting a pregnancy.
Infradian rhythms, therefore, respond to context cues, not precise timing cues. Unlike the circadian system, which maintains its rhythm in the absence of external light because daily timing is essential for survival, the menstrual cycle is meant to adjust when environmental conditions are unfavourable. A change in timing is therefore adaptive rather than pathological.
DISCLAIMER ABOUT FACE-PREFERENCE STUDIES
Some A-level websites list Penton-Voak et al. (1999) and similar face-preference studies as evidence for exogenous zeitgebers (EZs) in the menstrual cycle. This is incorrect.
These studies show changes in attraction and behaviour that track internal hormonal phases, providing evidence for endogenous pacemakers (EPs) rather than external influences. In fact, they demonstrate the opposite of an EZ effect: behaviour changes due to internal timing rather than environmental cues.
Students should also be aware that replication of face-preference effects has been inconsistent, and the strength of these findings is debated in the current research literature.
ULTRADIAN RHYTHMS
ULTRADIAN RHYTHMS
Ultradian rhythms are biological cycles that repeat multiple times within 24 hours.
Ultradian rhythms are biological cycles that repeat multiple times within 24 hours. They organise short-interval patterns of activity in systems that cannot operate at a constant output. Many physiological processes work best in bursts, followed by brief periods of recovery, and these alternating phases form the basis of ultradian timing.
One prominent example is hormone pulsatility. Hormones such as cortisol, growth hormone and testosterone are not released steadily; they are released in regular pulses. These pulses are essential because a constant stream of hormones would rapidly desensitise receptors and flatten the system’s responsiveness. The endocrine system uses ultradian timing to maintain sensitivity, recalibrate set-points and coordinate metabolic and reproductive processes.
Ultradian patterns also appear in attention and cognitive performance. Humans do not maintain stable concentration for hours; instead, the nervous system naturally alternates between periods of high focus and brief reductions in alertness. This rhythm, often described in cognitive neuroscience as the Basic Rest–Activity Cycle (BRAC), reflects fluctuations in neural firing thresholds, prefrontal resource use and arousal regulation. It represents the brain's short-interval “work–rest” structure.
Energy regulation also follows ultradian timing. Hunger and satiety cycles, glucose oscillations and gastrointestinal activity move through repeating phases across the day. These rhythms coordinate digestive activity with metabolic demands, ensuring that the system does not overshoot or undershoot energy availability.
Even immune activity and body temperature show ultradian fluctuations, with recurring rises and dips that support tissue repair, thermoregulation and inflammatory balance. These cycles occur because many biological systems rely on periods of activation followed by short recovery windows to maintain efficiency and prevent overload.
In short, ultradian rhythms manage the repeating biological work cycles that occur many times each day. They reflect the fact that internal systems operate in patterns of activation, feedback and restoration, rather than running continuously, and they allow the body to maintain responsiveness, stability and energetic efficiency across the 24-hour day.
THE SLEEP CYCLE AS AN EXTENDED EXAMPLE OF AN ULTRADIAN RHYTHM
The sleep cycle is a clear example of an ultradian rhythm because it repeats several times within 24 hours. Rather than being one uniform state, sleep follows a structured 90-minute sequence that moves through progressively deeper NREM stages before shifting into REM sleep, and this pattern recurs throughout the night.
AWAKE:
Brain activity is faster, dominated by beta waves (alertness) and alpha waves (relaxation).
LIGHT SLEEP N1:
As you fall asleep, alpha waves are replaced by slower theta waves, marking the light transition into sleep.
DEEP SLEEP N2:
Brainwaves: Theta waves continue to dominate, but the brain activity is interrupted by sudden bursts of electrical activity called sleep spindles and large spikes called K complexes.
Function: These bursts may help the brain disconnect from the external environment and consolidate memories.
RESTORATIVE SLEEP N3 Stage (DEEP SLOW WAVE SLEEP)
• Brainwaves: Characterised by delta waves, which are the slowest and have the highest amplitude, according to Wikipedia and SleepSpace.
• Function: This stage is critical for physical restoration, cell repair, and memory consolidation.
REM (Rapid Eye Movement) Sleep
Brainwaves: Brain activity becomes more similar to when you are awake, with alpha and beta waves present. The cycle has entered REM sleep, where brain activity becomes rapid and wake-like while the body undergoes REM atonia, a temporary paralysis that prevents acting out dreams..Function: This is when most vivid dreaming occurs and is also essential for learning and emotional processing
Across the night, the brain moves repeatedly through this NREM → REM sequence, usually four to six times, demonstrating the short-interval patterning characteristic of ultradian rhythms.
FURTHER READING:https://psychscenehub.com/psychinsights/neurobiology-sleep/
BRAIN WAVES IN THE FOUR STAGES OF SLEEP
.
BRAIN WAVES AND EEGS
Brain waves are essential for measuring sleep because each sleep stage has a unique pattern of electrical activity. These patterns are recorded with an EEG.
Frequency tells us how fast the waves are (measured in Hz).
Amplitude tells us how tall the waves are, which reflects how many neurons are firing together.
Synchronised firing means large groups of neurons fire at the same time in a steady rhythm. This happens when you are drowsy or in deep sleep because the brain is no longer jumping rapidly between thoughts or processing a lot of information.
Unsynchronised firing means neurons are firing in many different directions at once. This happens when you are awake or in REM sleep, because thinking is fast, varied and unpredictable, so the brain’s activity becomes messy and irregular.
Because each sleep stage has its own combination of frequency, amplitude and synchrony, brain waves are the only reliable way to identify where someone is in the sleep cycle.
Brain waves change as sleep deepens. When awake, activity is fast and mixed (alpha and beta waves). As you drift into Stage 1, the brain slows to theta waves. Stage 2 continues with theta waves, sleep spindles, and K complexes. Stage 3 is dominated by delta waves, which are slow and high in amplitude because neuronal firing becomes highly synchronised. REM sleep then switches back to fast, unsynchronised activity that resembles wakefulness.
These predictable changes allow researchers to accurately measure ultradian rhythms, such as the 90-minute sleep cycle.
THE 90-MINUTE SLEEP CYCLE AND ENDOGENOUS PACEMAKERS
Once a person falls asleep, the brain does not remain in a single state until morning. Instead, it follows a precise repeating pattern: a long period of NREM sleep followed by a shorter burst of REM sleep, then back to NREM, then REM again, and so on. This cycle lasts roughly 90 minutes and repeats 4 to 6 times per night. The NREM–REM alternation is one of the most reliable features of human sleep and appears consistently in standard sleep recordings. The entire sequence is generated internally by endogenous pacemakers that require no input from light, sound, or any other external cue.
The master pacemaker driving this rhythm sits in the pons, deep in the brainstem. Here, two opposing populations of neurons compete throughout the night. One population releases acetylcholine and pushes the brain toward REM sleep; the other releases serotonin and noradrenaline, maintaining NREM. Because each population actively inhibits the other, the moment one side weakens, the other gains control. This reciprocal inhibition produces a natural oscillator that alternates the brain between NREM and REM on a stable ninety-minute timetable.
When the serotonin and noradrenaline system is dominant, NREM takes over. Activity drops across large areas of the brain: the medial prefrontal cortex, the thalamus and much of the brainstem all become quiet. Energy use falls, slow delta waves sweep across the cortex, and the body shifts into deep restoration mode. During this time, the hippocampus replays the day’s experiences to the neocortex in an orderly fashion, strengthening declarative memories while the brain’s logical centres remain offline.
After about seventy to ninety minutes, the aminergic neurons begin to fatigue. As their firing slows, the acetylcholine-releasing REM-on neurons in the pons escape suppression and take control. At this point, everything reverses. The pons becomes highly active, driving the thalamus and cortex. Limbic structures such as the amygdala and hippocampus become intensely active, adding emotion to the state. Visual and sensory regions in the posterior cortex are strongly activated, generating vivid dream imagery. Meanwhile, the dorsolateral prefrontal cortex is deliberately deactivated, removing logical oversight and self-reflection so that dreams feel coherent but are often nonsensical.
The same pontine circuitry that triggers REM also sends inhibitory signals down the spinal cord, producing muscle atonia. This paralysis prevents the body from carrying out the vivid motor activity in the dream. After ten to sixty minutes, REM periods lengthen as the night progresses—acetylcholine levels drop again, the serotonin and noradrenaline neurons recover, and the brain transitions back into quiet NREM.
The cycle then repeats automatically until morning. The global deactivation of NREM, the selective activation and deactivation patterns of REM, the precise timing, the muscle paralysis, and the alternation between stages are all generated internally by the interaction between cholinergic and aminergic neurons in the pons and their downstream influence on the thalamus, limbic system, and cortex. Once sleep begins, no external zeitgeber is necessary: the brainstem’s chemical oscillator maintains the NREM–REM cycle independently. This is the endogenous pacemaker underlying the ultradian sleep cycle.
EXOGENOUS ZEITGEBERS THAT AFFECT THE ULTRADIAN NREM–REM CYCLE AND WHY
CAFFEINE – THE ADENOSINE BLOCKER
Caffeine works by blocking adenosine receptors in the brain. Adenosine is the chemical that accumulates across the day and drives the brain into deep, slow-wave sleep. When caffeine prevents adenosine from binding, the brain remains artificially aroused, reducing the amount of slow-wave sleep and delaying the onset of the first REM period. The result is lighter sleep and a disrupted first ultradian cycle.
ALCOHOL – SEDATIVE THEN REBOUND
Alcohol initially acts as a GABA-mimicking sedative, causing rapid sleep onset and increasing light NREM sleep in the first part of the night while almost completely suppressing REM. When the alcohol is metabolised several hours later, the brain compensates with a strong REM rebound. This produces intense, fragmented REM sleep in the second half of the night and destabilises the entire ultradian cycle.
NICOTINE – CONSTANT MINI-AROUSALS
Nicotine stimulates cholinergic arousal pathways and raises heart rate, making it harder to fall asleep and maintain deep NREM sleep. As nicotine levels drop during the night, withdrawal triggers repeated micro-arousals. These interruptions break up both slow-wave sleep and REM, preventing the brain from completing stable ultradian cycles.
CANNABIS / THC – REM SUPPRESSOR
THC suppresses REM sleep in a dose-dependent manner. The higher the intake, the more REM is reduced. When cannabis use stops, the brain responds with several nights of REM rebound characterised by vivid, intense dreaming. This creates irregular, unstable ultradian cycling until neural systems resynchronise.
BENZODIAZEPINES AND Z-DRUGS – GABA BOOSTERS
These medications enhance GABA’s inhibitory effects across the brain. As a result, Stage 2 sleep increases, but both deep slow-wave sleep and REM sleep decrease substantially. Because REM and deep NREM depend on precise patterns of excitation and inhibition in the brainstem, the extra global inhibition from these drugs disrupts the natural NREM–REM alternation.
OPIOIDS – DOUBLE SUPPRESSION
Opioids disturb the balance between cholinergic (REM-promoting) and aminergic (NREM-promoting) systems in the pons. Both slow-wave sleep and REM sleep are markedly reduced, and sleep becomes shallow and fragmented. The normal oscillation between ultradian stages is significantly weakened or absent.
STRESS AND CORTISOL – AROUSAL OVERDRIVE
Raised cortisol levels—whether from psychological stress or steroid medication—keep arousal circuits active and prevent the brain from settling into deep NREM. This delays the first REM episode, reduces overall REM sleep and lightens slow-wave sleep. The result is a distorted, irregular ultradian pattern.
BEDROOM TEMPERATURE – TOO WARM KILLS DEEP SLEEP
Slow-wave sleep requires a drop in core body temperature. If the room is too warm, the body cannot cool sufficiently to initiate deep NREM. As a result, Stage 3 sleep is reduced or absent in the first half of the night, and the ultradian cycle begins in a lighter, more fragile state.
NOISE AND LIGHT – MICRO-AROUSALS AND MELATONIN LOSS
Noise during sleep triggers microarousals that pull the sleeper out of deep NREM or REM sleep, interrupting the natural 90-minute cycle. Light exposure before or during sleep suppresses melatonin secretion and delays sleep onset, pushing the entire ultradian sequence later and making each cycle more fragmented.
LATE HEAVY MEALS AND LATE EXERCISE – METABOLIC AND THERMAL INTERFERENCE
Large meals or intense exercise shortly before bedtime raise core temperature and sympathetic nervous system activity. Because deep NREM depends on lowered body temperature and reduced physiological arousal, both activities delay or reduce slow-wave sleep, altering the early ultradian cycles.
BOTTOM LINE
The ninety-minute ultradian oscillator in the brainstem is endogenous and continues automatically throughout the night. However, these exogenous factors act as strong zeitgebers that alter how the cycle is expressed—making slow-wave sleep shallower, delaying REM, suppressing REM altogether or triggering rebound effects later. The stability and quality of ultradian sleep depend heavily on external conditions in the hours leading up to bedtime.
RESEARCH ON THE FOUR STAGES OF SLEEP
ENDOGENOUS PACEMAKER EVIDENCE — ULTRADIAN SLEEP STAGES
Aserinsky & Kleitman (1953):
Method: Used electroencephalography (EEG) and electro-oculography (EOG) to record brain activity and eye movements during sleep.
Findings: Identified rapid eye movement (REM) sleep and showed that REM and non-REM (NREM) phases alternate in a repeating pattern across the night.
Dement & Kleitman (1957):
Method: Conducted overnight polysomnography (PSG), including EEG, EOG and electromyography (EMG). Woke participants during different sleep stages to obtain dream reports.
Findings: Found a stable ~90-minute cycle of NREM to REM transitions; REM awakenings produced the highest dream recall.
Haider, Oswald & Adam (1970):
Method: Recorded full-night EEG in controlled sleep laboratories.
Findings: Slow-wave sleep dominated early cycles; REM sleep increased later in the night in a consistent, repeating pattern.
Shapiro et al. (1981):
Method: Monitored sleep using PSG before and after participants completed a 92-km ultramarathon.
Findings: Slow-wave sleep increased after extreme exertion, but the NREM→REM cycle remained unchanged.
Tucker et al. (2007):
Method: Used multi-night PSG recordings in a controlled laboratory to track sleep architecture.
Findings: Found significant individual differences in stage duration, but all participants maintained the same repeating ultradian cycle.
Randy Gardner Case (1964):
Method: After 264 hours awake, an EEG was used to measure recovery sleep across several nights.
Findings: There was a selective rebound of slow-wave sleep and REM sleep, not proportional repayment of total lost sleep.
Borbély (1982):
Method: Analysed EEG data and developed the Two-Process Model of sleep regulation.
Findings: Demonstrated that sleep pressure and circadian timing interact to produce the recurring NREM–REM structure.
EXOGENOUS ZEITGEBER EVIDENCE — ULTRADIAN SLEEP STAGES
Drapeau et al. (2006) — Caffeine:
Method: Administered 200 mg of caffeine or a placebo and monitored sleep using PSG.
Findings: Evening caffeine delayed sleep onset, reduced Stage 2 sleep, decreased slow-wave (delta) activity and delayed REM onset.
Full Drapeau et al 2006 article here
Ekirch et al. (Alcohol studies):
Method: Recorded sleep stages using EEG after controlled alcohol consumption.
Findings: Alcohol increased light N1 sleep, suppressed early-night REM and fragmented slow-wave sleep.
Campbell et al. (2018) — Cannabis/THC:
Method: Assessed chronic cannabis users and controls using PSG and actigraphy.
Findings: Cannabis reduced REM sleep and produced REM rebound during abstinence.
Roehrs et al. (1996) — Nicotine:
Method: Administered nicotine and monitored sleep through PSG.
Findings: Nicotine increased sleep latency, reduced slow-wave sleep and destabilised REM.
Nicholson et al. (1985) — Benzodiazepines:
Method: Examined sleep-stage effects of hypnotic medication with overnight EEG.
Findings: Benzodiazepines reduced slow-wave sleep, increased Stage 2 sleep and suppressed REM sleep.
Van den Heuvel et al. (1998) — Temperature:
Method: Manipulated ambient temperature before sleep and monitored sleep using PSG.
Findings: Cooler temperatures increased slow-wave sleep; heat suppressed slow-wave sleep and delayed REM.
Steiner & Halberg (1975) — Noise:
Method: Exposed participants to varying levels of environmental noise while recording sleep with EEG.
Findings: Noise caused frequent micro-arousals, interrupted REM periods and forced returns to lighter NREM stages.
Bertrand et al. (1993) — Stress:
Method: Measured sleep using PSG after inducing psychological stress.
Findings: Acute stress delayed REM onset and reduced REM density; chronic stress reduced slow-wave sleep
CHALLENGING / QUALIFYING EVIDENCE
Some individuals show high resilience to certain zeitgebers (e.g., caffeine non-responders).
Drug effects can vary by tolerance, genetics, and chronic use.
Environmental disruptions often co-occur (noise, stress, and light), making it hard to isolate a single zeitgeber.
However, none of these counterexamples remove the core ultradian rhythm—they only distort it.
SUMMARY
There is strong evidence that exogenous zeitgebers—caffeine, alcohol, nicotine, cannabis, benzodiazepines, opioids, temperature, noise and stress—alter the structure and expression of REM and NREM sleep stages.
But crucially: They can distort, fragment, delay or shift stages…BUT THEY DO NOT ABOLISH THE 90-MINUTE ULTRADIAN CYCLE, which always reasserts itself
EVALUATION OF THE ULTRADIAN SLEEP CYCLE
ENDOGENOUS PACEMAKERS CLEARLY GENERATE THE 90-MINUTE ULTRADIAN CYCLE
A significant strength of the endogenous explanation is the sheer volume of converging evidence showing that the NREM–REM alternation is internally generated and does not depend on any external zeitgeber. Once sleep begins, the brainstem—particularly the pontine REM-on (acetylcholine-based) and REM-off (serotonin/noradrenaline-based) systems—functions as a self-contained oscillatory circuit. This reciprocal inhibition forms a biological “switch” that naturally alternates between NREM and REM at approximately 90-minute intervals.
Temporal isolation studies provide some of the most substantial evidence. Individuals sleeping in environments completely stripped of external cues (no light cycles, no clocks, no temperature changes, no social interaction) still show the same 90-minute NREM → REM → NREM → REM sequence, with minimal drift. If the cycle depended on external stimuli, its timing would collapse or vary unpredictably under such conditions. Instead, it remains remarkably stable, indicating an internal pacemaker.
Lesion studies strengthen this claim further. When researchers selectively destroy REM-generating pontine nuclei in animals, REM sleep disappears entirely, and the ultradian cycle breaks down. Crucially, no external input can restore the cycle, demonstrating that the architecture of NREM–REM cycling is located inside the brainstem and not imposed from outside. This is a strong causal inference: without the neural generator, the rhythm does not exist.
Pharmacological evidence provides additional support. Drugs such as alcohol, SSRIs and benzodiazepines can suppress REM or flatten sleep architecture for several hours. Yet, once these chemical effects wear off, the NREM–REM sequence reappears exactly where the internal clock predicts it should, rather than restarting from the beginning or adjusting to environmental conditions. This “snap-back” phenomenon strongly implies that the timing mechanism continues to run beneath the drug suppression, again pointing to a robust endogenous oscillator.
Taken together, these findings form a powerful argument: the ultradian sleep cycle shows persistence in isolation, disruption by structural damage, and rebound according to internal timekeeping, all of which are the defining characteristics of an endogenous biological rhythm. The consistency, precision and resilience of the 90-minute cycle make it one of the clearest examples in human physiology of a pacemaker-driven process that operates independently of environmental influences.
BRAINSTEM DAMAGE ABOLISHES THE ULTRADIAN CYCLE
A second major strength of the endogenous explanation comes from lesion research showing that the ultradian sleep cycle cannot exist without its neural generator. When the pons or its associated REM-on and REM-off nuclei are damaged, the entire NREM–REM alternation collapses. Animals with pontine lesions do not merely show altered timing or reduced REM; the cycle is abolished outright. This is critical because if exogenous zeitgebers such as light, sound, feeding schedules or temperature were capable of generating the rhythm, then the cycle should re-emerge once these cues are reintroduced. In reality, no external stimulation can restore NREM–REM cycling after the pontine circuitry is destroyed. The rhythm does not reappear, does not drift, and does not compensate — it simply ceases to exist. This demonstrates a strong causal relationship: the generator lies inside the brainstem, not outside it. Exogenous influences can modulate the rhythm’s expression, but they cannot produce its fundamental oscillation. The fact that the cycle exists only when the brainstem circuitry is intact provides some of the most decisive evidence that the ultradian sleep cycle is driven by an endogenous pacemaker rather than imposed or generated by environmental cues.
MEASUREMENT LIMITATIONS RESTRICT THE VALIDITY OF ULTRADIAN RESEARCH
A major methodological issue concerns the tools used to measure ultradian rhythms. Polysomnography is the gold standard, but it involves sleeping in a laboratory with electrodes attached to the scalp and body. This environment triggers the well-documented “first-night effect”, where individuals sleep more lightly, show altered sleep architecture and produce atypical ultradian cycles. Even after adaptation nights, the artificial setting may influence natural sleep patterns. PSG also requires small sample sizes due to cost and complexity, making it difficult to generalise findings. Home EEG devices avoid this problem but tend to have lower signal resolution, blurring the boundaries between NREM stages and reducing the accuracy of REM transition detection. These limitations mean that many studies may not capture an individual’s true ultradian rhythm, restricting both ecological validity and reliability. Consequently, while laboratory evidence for endogenous oscillators is strong, the exact structure and timing of the cycle in everyday contexts is less firmly established.
EXOGENOUS FACTORS CAN DISTORT, BUT NOT DRIVE
Although the 90-minute ultradian cycle is generated internally by the reciprocal interaction of cholinergic REM-on neurons and aminergic REM-off neurons in the pons, there is extensive evidence showing that external factors can distort how the cycle is expressed. Substances such as alcohol, cannabis, benzodiazepines, SSRIs, caffeine and nicotine, along with acute or chronic stress, can suppress, delay or fragment particular stages for several hours at a time. Alcohol, for example, removes REM almost entirely from the early night and replaces it with light NREM; caffeine blocks adenosine and prevents entry into deep slow-wave sleep; nicotine repeatedly triggers micro-arousals as blood levels fluctuate; SSRIs suppress REM for as long as they are in the system; and stress elevates cortisol and sympathetic activity, making both deep NREM and REM harder to initiate. Yet in every one of these cases, the fundamental 90-minute timing mechanism continues to run beneath the disturbance. Once external interference ends—whether through drug metabolism or resolution of the stressor—the ultradian cycle returns immediately, and always at the phase the internal timer predicts. Often, the stage that was suppressed returns with a rebound effect, such as the intense REM rebound seen after alcohol or cannabis. This pattern demonstrates that exogenous influences can modify the expression of the cycle, but they do not generate or time it. They behave like static over a radio signal: capable of degrading the output, but incapable of producing the pattern itself.
APPLICATIONS TO THE REAL WORLD
This distinction between endogenous timing and exogenous distortion has essential real-world consequences. Because each sleep stage serves critical functions—slow-wave sleep supporting immune regulation, metabolic stability and physical restoration, and REM supporting emotional regulation and limbic recalibration—anything that persistently suppresses these stages carries predictable risks. For example, long-term REM suppression, whether caused by SSRIs, chronic cannabis use, alcohol-dependent sleep, or disrupted schedules, can blunt emotional processing, increase amygdala reactivity and reduce the brain’s ability to regulate affect. This is why some individuals experience paradoxical increases in anxiety or worsening low mood when REM sleep is chronically reduced. Likewise, repeated suppression of slow-wave sleep through late caffeine use, nicotine, high evening stress, or inadequate temperature reduction contributes to poorer glucose regulation, reduced immune function, heightened pain sensitivity, and reduced next-day cognitive performance.
Understanding these mechanisms provides behavioural guidance. If the internal 90-minute cycle cannot be altered externally, then the only practical way to protect sleep architecture is to minimise the exogenous factors that distort it. This is why caffeine consumed after mid-afternoon continues to impair slow-wave sleep long after the user feels “sober,” why smoking before bed leads to a night of broken NREM and REM, and why alcohol-induced “sleep” is physiologically closer to sedation than to actual restorative sleep. It also clarifies why benzodiazepines and Z-drugs may help initiate unconsciousness but do not replicate the natural architecture of sleep: they suppress precisely the stages the brain needs most. Recognising the distinction between the underlying oscillator and the factors that interfere with it highlights the importance of sleep hygiene. It helps individuals understand that what they ingest or do in the hours before bed has measurable, predictable and long-lasting consequences for emotional stability, physical health and cognitive performance.
INDIVIDUAL DIFFERENCES LIMIT THE GENERALISABILITY OF THE 90-MINUTE MODEL
A further evaluative point concerns the considerable individual differences in ultradian sleep architecture, which limit the universality of the “fixed” 90-minute model. Although the average adult cycles between NREM and REM roughly every ninety minutes, the actual duration of the cycle varies widely: some individuals have cycles closer to seventy minutes, others closer to one hundred, and these timings can remain stable across years. Even within the same individual, cycle length shifts with illness, hormonal state, stress and accumulated sleep debt. These differences imply that while the oscillator is endogenous, its specific periodicity is not identical across the population and cannot be applied as a rigid template. This variability raises methodological concerns in sleep research because laboratory averages may conceal meaningful subgroup differences. As a result, any explanatory model of ultradian rhythms must acknowledge that the 90-minute cycle is a population mean, not a universal biological constant.
AGEING AND DEVELOPMENTAL CHANGES ALTER ULTRADIAN ARCHITECTURE
Another limitation is that ultradian sleep patterns change dramatically with age, indicating that the underlying pacemaker is biologically malleable rather than fixed. Infants show ultradian cycles of roughly fifty minutes with REM dominating the night; adolescents show increased slow-wave sleep and longer NREM phases; and older adults experience marked reductions in slow-wave sleep alongside shorter, more fragmented REM episodes. By late adulthood, deep NREM may almost disappear entirely, and REM becomes more unstable, suggesting that the strength and coordination of pontine oscillators weaken over time. These changes highlight the challenge of generalising findings from young adults (who make up the majority of laboratory samples) to the broader population. The developmental plasticity of the ultradian rhythm provides essential nuance: the pacemaker is endogenous, but its strength, expression and stability reflect biological age and neurochemical integrity.
SOCIAL AND LIFESTYLE FACTORS DISTORT EXPRESSION OF THE RHYTHM
Although exogenous factors cannot initiate the cycle, their expression remains highly sensitive to modern environmental conditions. Artificial light exposure late in the evening delays sleep onset and compresses early slow-wave sleep; screen-based blue light pushes the first REM period deeper into the night; irregular social schedules create “social jet lag”, fragmenting the ultrasonic oscillation; and inconsistent wake times weaken the coupling between circadian and ultradian systems. These effects do not challenge the existence of an endogenous pacemaker, but they illustrate how modern routines can mask, distort or destabilise its output. Because sleep research is typically conducted under controlled laboratory conditions, many studies underestimate the extent to which real-world behaviour alters the observed structure of the cycle. Thus, while the oscillator is internal, its translation into actual sleep patterns is fundamentally shaped by lifestyle.
WHAT STUDENTS NEED TO KNOW ABOUT EXAM QUESTIONS
AQA has not yet made students choose a specific biological rhythm in any of the biological rhythm questions. The questions have been generic and used the broad phrase 'biological rhythms,' allowing students to choose whichever rhythm they want to write about. In this scenario, they should still have one rhythm prepared in depth so they can deliver a complete answer when needed. In practice, most students use the sleep–wake cycle because it is the clearest and has the strongest research base, but any biological rhythm is acceptable if it is accurate and well supported.
Importantly, this does not mean you can ignore the two other biological rhythms, because the specification requires knowledge of three named rhythms, so students must know the definitions and distinguishing features of circadian, infradian, and ultradian rhythms. These may appear in short-answer or multiple-choice questions, and there is no guarantee that AQA will never set a 16-marker on a specific rhythm.
The core of this topic is understanding how biological rhythms are controlled. Internal timing is driven by endogenous pacemakers such as the SCN, the pineal gland, and melatonin release. External timing is driven by environmental cues such as light, temperature, and social routines. The exam focus is not on memorising three separate rhythms but on explaining how these internal and external systems work together to maintain regular biological timing.
This links to the central conceptual issue in the topic: are biological rhythms primarily driven from within the body, or do they depend heavily on environmental input? Real examples make this clearer. Blind individuals still show daily patterns, suggesting strong internal timing, whereas shift workers struggle with artificial light at night, showing the strength of external cues. These examples anchor the “nature versus nurture” discussion that examiners expect students to address when evaluating the topic.
Students need to know which aspects of a rhythm are internally controlled and which are shaped by the environment. They should be able to organise research accordingly: studies such as Siffre's and Folkard's demonstrate endogenous control in isolation, while research by Czeisler and others shows how external cues can shift or stabilise the rhythm. Disruption studies then apply the same logic to everyday behaviour by showing how misaligned cues lead to desynchronisation.
In exams, the command words determine the emphasis. “Outline” and “describe” require accurate AO1 detail and key research. “Explain” and “discuss” need a clear account of how internal and external factors interact, supported by evaluation and real-world application. The most effective way to approach this topic is to treat Biological Rhythms as a single integrated framework: the rhythms provide the examples, and the mechanisms explain how those patterns are maintained, controlled, and sometimes disrupted.
The emphasis is on how biological rhythms are controlled—specifically, the interaction between endogenous pacemakers (internal biological clocks, such as the SCN and melatonin release) and exogenous zeitgebers (external environmental cues, such as light, temperature, and social routines). Students must understand that both systems work together to keep internal bodily processes synchronised with the external environment.
Students should also be aware of how this topic is questioned and how to structure their knowledge effectively.
Questions on biological rhythms vary in focus.
Outline and evaluate research into biological rhythms – focuses on studies and evidence.
Discuss the roles of endogenous pacemakers and exogenous zeitgebers in the control of biological rhythms—emphasising explanations and mechanisms.
Discuss disruption of biological rhythms (e.g., shift work, jet lag) – focuses on application to real-world behaviour.
To prepare, students should organise their understanding so they can adapt quickly to any of these question styles. A clear system is as follows:
Define and describe each type of rhythm (circadian, infradian, ultradian), but have one rhythm—usually the sleep–wake cycle—ready to use in detail.
Link mechanisms to rhythms. Know which aspects of the rhythm are controlled internally (endogenous pacemakers such as the SCN, pineal gland, melatonin) and which are shaped by external cues (exogenous zeitgebers such as light, temperature, or social factors).
Organise research logically. Separate studies into those that demonstrate endogenous control (e.g., Siffre, Folkard et al.) and those that show the influence of exogenous factors (e.g., Czeisler et al., light exposure studies).
Apply knowledge to disruption. Understand how jet lag, shift work, and artificial light alter exogenous cues and lead to desynchronisation of the body’s internal clock.
AQA expects:
AO1 (knowledge): Clear definitions, examples, and biological detail (SCN, melatonin, light cues).
AO2 (application): Use scenarios to link theory to real-life contexts (e.g., shift work, jet lag).
AO3 (evaluation): Reference to key studies — Siffre (1962, 1975), Folkard (1985), Czeisler (1999) — and discuss validity, generalisability, and real-world implications
Respond to command words.
Outline/Describe → AO1: factual detail, processes, and key research.
Explain/Discuss → AO2/AO3: interaction between systems, real-life examples, and critical evaluation.
When you are asked a 16-mark question on circadian rhythms, they will either ask you:
Discuss research into biological rhythms (16). This means you need to use GRAVER to evaluate the research.
Discuss the role of exogenous zeitgebers (external) on biological rhythms (16). This will involve you creating arguments about the extent to which exogenous zeitgebers play a role, or whether endogenous pacemakers play a greater role in influencing Infradian rhythms.
Discuss the role of endogenous pacemakers (internal) on biological rhythms (16). This will involve you creating arguments about the extent to which endogenous pacemakers play a role, or whether exogenous zeitgebers play a greater role in influencing Infradian rhythms. You can use some of the points from the essay above to help you with this.
In summary, students should view the topic of Biological Rhythms as a single framework of ideas: biological rhythms provide the context, and endogenous pacemakers and exogenous zeitgebers explain. A successful answer depends on selecting the appropriate rhythm, linking it to its controlling mechanisms, and clearly and coherently applying relevant evidence to the specific question asked..
EXAMINATION QUESTIONS
Here are typical AQA-style exam questions on Biological Rhythms — covering circadian, infradian, and ultradian rhythms, as well as endogenous pacemakers and exogenous zeitgebers. They are phrased in the same style and use the same command terms as in AQA past papers.
SHORT QUESTIONS ON BIOLOGICAL RHYTHMS
2. The human female menstrual cycle is an example of one type of biological rhythm; it is called a:
A circadian rhythm
B infradian rhythm
C ultradian rhythm (1 mark)
What is meant by a biological rhythm?
A) A learned routine
B) A repeating pattern of biological activity
C) A reflex
D) A hormone change only ( 1 mark)
The central endogenous pacemaker is the:
A) Cerebrum
B) Suprachiasmatic nucleus (SCN)
C) Adrenal gland
D) Hippocampus
E) Cerebral cortex ( 1 mark)
Which of the following is an exogenous zeitgeber?
A) Melatonin
B) Light
C) Cortisol
D) Serotonin
E) Hunger ( 1 mark)
Write one short sentence for each term in the context of biological rhythms. ( 1 mark each)
A) Endogenous pacemaker
B) Exogenous zeitgeber
C) Circadian rhythm
SHORT QUESTIONS ON CIRCADIAN RHYTHMS
Describe how light acts as an exogenous zeitgeber in humans. ( 2 marks).
When light enters the eyes in the morning, melatonin levels:
A) Increase B) Decrease C) Stay the same D) Double ( 1 mark)When light leaves the eyes in the evening, serotonin levels: ( 1 mark)
A) Increase B) Decrease C) Stay the same D) DoubleWhich of the following is not a circadian rhythm? ( 1 mark)
A) Sleep–wake cycle B) Menstrual cycle C) Puberty D) REM/NREM sleep cycleWrite one short sentence for each term in the context of circadian rhythms. ( 1 mark each)
A) Suprachiasmatic nucleus (SCN)
B) Circadian rhythm
C) The sleep Wake cycle
D) Entrainment
Name one circadian endogenous pacemaker and describe its role. ( 2 marks)
Name one exogenous zeitgeber and describe how it influences the circadian cycle. ( 3 marks)
What happens when the SCN is damaged or cut off from light cues? ( 3 marks)
What gland releases melatonin, and when? ( 3 marks)
Give one real-world example of circadian disruption and its consequences. ( 3 marks)
Identify two examples of circadian rhythms in humans. (2 marks)
Name one endogenous pacemaker and one exogenous zeitgeber. (2 marks)
State the primary function of the suprachiasmatic nucleus (SCN). (3 marks)
Outline one effect of disruption to circadian rhythms. (3 marks)
Define what is meant by a biological rhythm. (4 marks)
Outline one or more examples of ultradian rhythms.( 4 marks)
Describe biological rhythms (4 marks).
Explain the difference between circadian rhythms, infradian rhythms and ultradian rhythms and give an example for each. ( 6 marks)
SHORT QUESTIONS ON INFRADIAN RHYTHMS
An infradian rhythm lasts:
A) Less than 24 hours B) About 24 hours C) Longer than 24 hours D) 90 minutes ( 1 mark)Which of the following is an example of an infradian rhythm?
A) REM/NREM cycle B) Menstrual cycle C) Core body temperature D) Alertness cycle ( 1 mark)Seasonal affective disorder (SAD) is linked to:
A) Light exposure changes melatonin . B) REM sleep . C) Dopamine surges only D) Hunger signals ( 1 mark)Infradian rhythms repeat:
A) Several times per day
B) Once every 24 hours
C) Less than once per day
D) Exactly once per week ( 1 mark)The menstrual cycle is controlled by the:
A) HPA axis
B) HPO axis
C) SCN only
D) Retina and pineal gland ( 1 mark)Which factor can act as an exogenous cue affecting infradian rhythms?
A) Stress
B) Oestrogen pulses
C) GnRH release
D) Melatonin breakdown ( 1 mark)Write one short sentence for each term.in the context of biological rhythms. ( 1 mark each)
A) Infradian rhythm
B) The Menstrual cycle or SAD
C) Exogenous zeirgerbers of the menstrual cycle
D) Endogenous pacemaker of the menstrual cycle
SHORT QUESTIONS ON ULTRADIAN RHYTHMS
Ultradian rhythms have cycles that:
A) Last longer than 24 hours
B) Take exactly 24 hours
C) Occur more than once in 24 hours
D) Occur once per year ( 1 mark)The REM/NREM cycle typically lasts: ( 1 mark)
A) 15 minutes B) 30 minutes C) 90 minutes D) 6 hoursREM sleep is associated with:
A) Slow-wave Delta waves
B) Dreaming and rapid eye movements
C) Immobility of the brain
D) Higher melatonin releaseWhich of the following is an ultradian rhythm? ( 1 mark)
A) Menstrual cycle
B) Stages of sleep
C) Puberty
D) Daily cortisol patternDuring NREM stage 3/4 sleep, the brain shows: ( 1 mark)
A) Beta waves
B) Alpha waves
C) Delta waves
D) REM burstsWrite one short sentence for each term in the context of biological rhythms. ( 1 mark each)
A) Ultradian rhythm
B) The four stages of the Sleep cycle/REM and NREM sleep
C) Endogenous pacemakers
E) Exogenous zeitgebers of the
ENDOGENOUS PACEMAKERS: SHORT QUESTIONS
The primary endogenous pacemaker in mammals is the:
A) Retina B) SCN C) Adrenal gland D) CerebellumThe SCN communicates with the pineal gland to control:
A) Cortisol release B) Melatonin release C) Dopamine D) Glucose levels ( 1 mark)A free-running rhythm is a rhythm that:
A) Requires light
B) Runs without external cues
C) Is controlled by diet
D) Cannot be measured ( 1 mark)The SCN receives light information from:
A) Hair cells
B) The optic nerve
C) Sweat glands
D) The hippocampus ( 1 mark)If the SCN is damaged, sleep–wake cycles:
A) Become random
B) Strengthen
C) Occur twice per day
D) Stop completely ( 1 mark)Endogenous pacemakers are best described as:
A) External cues that reset rhythms
B) Neural systems that generate rhythms internally
C) Learned responses
D) Digestive triggers ( 1 mark)Write one short sentence for each term. ( 2 marks each)
A) Endogenous pacemaker
B) SCN (suprachiasmatic nucleus)
C) Pineal gland
D) Melatonin
D) Free-running rhythm
EXOGENOUS ZEITGEBERS — SHORT QUESTIONS
The strongest exogenous zeitgeber for humans is:
A) Light B) Sound C) Food D) Exercise ( 1 mark)Social cues act as zeitgebers by:
A) Altering DNA
B) Providing predictable routines
C) Increasing melatonin directly
D) Blocking SCN activity ( 1 mark)Which of the following is not an exogenous zeitgeber?
A) Temperature B) Light C) Mealtimes D) Melatonin ( 1 mark)Which statement is true about exogenous zeitgebers?
A) They operate entirely inside the body
B) They come from outside the organism
C) They replace endogenous pacemakers
D) They only affect animals, not humans ( 1 mark)Light resets the SCN by:
A) Blocking REM sleep
B) Adjusting melatonin signals
C) Increasing hunger hormones
D) Activating the amygdala ( 1 mark)Jet lag occurs because:
A) Endogenous pacemakers stop working
B) External cues no longer match internal timing
C) The brain forgets day and night
D) Ultradian rhythms slow down ( 1 mark)Write one short sentence for each term. ( 2 marks each)
A) Exogenous zeitgeber
B) Light
C) Social cues
D) Temperature changes
C) Entrainment (external)
APPLICATION QUESTIONS ON BIOLOGICAL RHYTHMS
Scenario-based short applications. Read the items and then answer the questions that follow.
A group of nurses work alternating day and night shifts. Explain how their sleep–wake cycles may become disrupted. (6 marks)
Ella flies from London to Japan and struggles to fall asleep for several nights. Explain why this happens, referring to endogenous pacemakers and exogenous zeitgebers. (6 marks)
“Sam is a police officer. She has just started working the night shift, and after a week, she finds that she has difficulty sleeping during the day and is becoming tense and irritable. Sam is also worried that she is less alert during the night shift itself.” Using your knowledge of endogenous pacemakers and exogenous zeitgebers, explain Sam’s experiences. ( 6 marks)
Explain one way in which research into circadian rhythms can be applied to improve human wellbeing. (6 marks)
Julia complains that her baby is sleeping all day and keeping her awake all night.. Using your knowledge of research into exogenous zeitgebers, discuss what Julia could do to encourage her baby to sleep more at night. (8 marks)
Explain how endogenous pacemakers and exogenous zeitgebers interact to regulate biological rhythms. (6 marks)
OUTLINE/DESCRIBE/AO1 QUESTIONS ON BIOLOGICAL RHYTHMS (6 marks)
Outline the role of endogenous pacemakers in controlling biological rhythms. (6 marks)
Outline the role of exogenous zeitgebers in controlling biological rhythms. (6 marks)
Describe biological rhythms
EVALUATE QUESTIONS ON BIOLOGICAL RHYTHMS (10 marks)
Evaluate research into biological rhythms. (10 marks)
Evaluate how disruption of biological rhythms (e.g., shift work or jet lag) can affect human behaviour. (10 marks)
ESSAY QUESTIONS ON BIOLOGICAL RHYTHMS (16 marks)
Longer essay questions (AO1 + AO3 and sometimes AO2 combined)
“Endogenous pacemakers and exogenous zeitgebers influence Biological Rhythms”: Outline the difference between endogenous pacemakers and exogenous zeitgebers. Use examples in your answer. (Total 16 marks)
Discuss research into biological rhythms. This means you need to use GRAVER to evaluate the research. (Total 16 marks)
Discuss the role of exogenous zeitgebers (external) on biological rhythms. This will involve you creating arguments about the extent to which exogenous zeitgebers play a role, or whether endogenous pacemakers play a greater role in influencing Infradian rhythms. (Total 16 marks)
Discuss the role of the endogenous pacemaker (internal) on biological rhythms. This will involve you creating arguments about the extent to which endogenous pacemakers play a role, or whether exogenous zeitgebers play a greater role in influencing Infradian rhythms. You can use some of the points from the essay above to help you with this. (Total 16 marks)
Outline and evaluate the role of endogenous pacemakers and exogenous zeitgebers in the control of biological rhythms. (16 marks)
Outline and evaluate research into biological rhythms. (16 marks)
Discuss the role of endogenous pacemakers in the control of one or more biological rhythms. (Total 16 marks)
ACTIVITIES
CIRCADIAN CLOCK MATCH ACTIVITY
Instructions:
Match each behaviour or physiological event below to the correct time on the 24-hour circadian clock.
Then discuss the extension questions with your group
TIMINGS
02:00
04:30
06:45
07:30
08:30
09:00
10:00
12:00
14:30
15:30
17:00
18:30
19:00
21:00
22:30
Make it stand out
Whatever it is, the way you tell your story online can make all the difference.
ANSWERS
TIME BEHAVIOUR/PHYSICAL EVENT
02:00 Deepest sleep
04:30 Lowest body temperature
06:45 Sharpest rise in blood pressure
07:30 Melatonin secretion stops
08:30 Bowel movement likely
09:00 Highest testosterone secretion
10:00 High alertness
12:00 (Noon)Midday reference point
14:30 Best coordination
15:30 Fastest reaction time
17:00 Greatest cardiovascular efficiency and muscle strength
18:30 Highest blood pressure
19:00 Highest body temperature
21:00 Melatonin secretion starts
22:30 Bowel movements suppressed
SORTING ACTIVITY: PACEMAKERS OR ZEITGEBERS?
Instructions: Below is a mixed list of biological and environmental factors.
Decide whether each one is an Endogenous Pacemaker (internal clock or mechanism) or an Exogenous Zeitgeber (external time cue).
Place each item into the correct column of the table.
Under “How it affects the sleep–wake cycle,” briefly describe its role (e.g., promotes wakefulness, triggers sleep, synchronises timing, etc.).
·Light
Temperature
Social cues
Suprachiasmatic nucleus (SCN)
Pineal gland
Increase in melatonin
Decrease in melatonin
Increase in serotonin
Decrease in serotonin
Hormone secretion patterns
Alertness
Heartbeat
Blood pressure
Body temperature
Mealtimes
Work schedules
Exercise and physical activity
Artificial light
Jet lag
Shift work
Geographical location
Season
Age